Abstract
Cocaine addiction is often modeled in experimental paradigms where rodents learn to self-administer (SA) the drug. However, the extent to which these models replicate the functional alterations observed in clinical neuroimaging studies of cocaine addiction remains unknown. We used magnetic resonance imaging (MRI) to assess basal and evoked brain function in rats subjected to a prolonged, extended-access cocaine SA scheme. Specifically, we measured basal cerebral blood volume (bCBV), an established correlate of basal metabolism, and assessed the reactivity of the dopaminergic system by mapping the pharmacological MRI (phMRI) response evoked by the dopamine-releaser amphetamine. Cocaine-exposed subjects exhibited reduced bCBV in fronto-cortical areas, nucleus accumbens, ventral hippocampus, and thalamus. The cocaine group also showed an attenuated functional response to amphetamine in ventrostriatal areas, an effect that was significantly correlated with total cocaine intake. An inverse relationship between bCBV in the reticular thalamus and the frontal response elicited by amphetamine was found in control subjects but not in the cocaine group, suggesting that the inhibitory interplay within this attentional circuit may be compromised by the drug. Importantly, histopathological analysis did not reveal significant alterations of the microvascular bed in the brain of cocaine-exposed subjects, suggesting that the imaging findings cannot be merely ascribed to cocaine-induced vascular damage. These results document that chronic, extended-access cocaine SA in the rat produces focal fronto-cortical and striatal alterations that serve as plausible neurobiological substrate for the behavioral expression of compulsive drug intake in laboratory animals.
Similar content being viewed by others
INTRODUCTION
Chronic cocaine use produces long-lasting neurobiological changes that are thought to underlie the loss of control over drug intake that defines cocaine dependence (Koob et al, 1998). Human neuroimaging studies have started to shed light on the nature of these changes and their relationship with specific behaviors or symptoms. Reduced frontostriatal perfusion and metabolism in abstinent cocaine abusers have been reported by multiple investigators (Strickland et al, 1993; London et al, 1999; Volkow et al, 1992). The disrupted function of frontal regions has been linked to the persistent neuropsychological deficits and impaired control over drug taking that frequently triggers relapse (Strickland et al, 1993; Kalivas, 2004). Positron emission tomography (PET) studies with selective D2 dopamine (DA) ligands have demonstrated that subjects with cocaine addiction show persistent reduction in D2 DA receptor availability (Volkow et al, 1993; Martinez et al, 2004) and decreased dopaminergic responsivity in the nucleus accumbens and other components of the ‘reward circuit’ (Volkow et al, 1997), consistent with a decreased sensitivity to natural reinforcers observed in these subjects (Volkow et al, 2007). Recent research suggests that altered functional connectivity of catecholamine circuits may underlie the impaired inhibition of cortical function observed in cocaine abusers, a finding that portrays novel pathways for the neuroadaptational processes associated with addictive states (Tomasi et al, 2010; Gu et al, 2010).
Cocaine abuse is often modeled preclinically in experimental paradigms where rats are trained to self-administer (SA) the drug. By employing different SA patterns, experimenters have been able to reproduce several hallmark features of drug addiction, including compulsive drug seeking (Vanderschuren and Everitt, 2004), uncontrolled drug use (Ahmed and Koob, 1998), and increased motivation to SA the drug (Paterson and Markou, 2003). These features make these models an experimental tool of excellent face validity to investigate the neuroplastic events associated with voluntary drug intake (Roberts et al, 2007). However, specific clinical correlates of cocaine addiction, such as the blunted DA responsivity of striatal areas observed in PET studies (Volkow et al, 1993; Martinez et al, 2004), do not appear to be adequately modeled by traditional short-term, limited-access cocaine SA paradigms, where instead ‘sensitized’ (ie, increased) dopaminergic responses are typically observed (Narendran and Martinez, 2008). Moreover, the extent to which these models replicate the multiple neurofunctional alterations observed in human neuroimaging studies remains unknown.
In the present study, we used magnetic resonance imaging (MRI) to map basal and evoked brain function in a rat model of cocaine SA. A prolonged (52 days), extended-access (12 h) SA protocol was employed to model the characteristics of high-dose, chronic cocaine abuse in humans (Gawin and Ellinwood, 1988; Briand et al, 2008). Repeated abstinence periods were introduced to minimize the acute toxic effects of the drug and to ensure sustained motivation to self-administer high doses of cocaine (Roberts et al, 2007). After a 10-day detoxification period, we measured microvascular basal cerebral blood volume (bCBV), an indirect indicator of resting brain function (Gaisler-Salomon et al, 2009; Small et al, 2004), and assessed the reactivity of dopaminergic system by mapping the functional response elicited by the DA-releaser amphetamine using a CBV-based pharmacological MRI (phMRI) protocol (Gozzi et al, 2010; Schwarz et al, 2004). Correlation analyses between resting (bCBV) and amphetamine-evoked (rCBV) responses were performed in an attempt to identify dysregulation in circuits that control the recruitment and functional responsiveness of specific brain areas. Finally, post-mortem histopathological examinations were carried out to assess the potential contribution of direct vascular and neurotoxic effects of prolonged cocaine SA to the imaging findings.
MATERIALS AND METHODS
Experiments were carried out in accordance with the Italian regulations governing animal welfare and protection. Protocols were also reviewed by a local animal care committee, in accordance with the guidelines of the Principles of Laboratory Animal Care (NIH publication 86–23, revised 1985).
Cocaine SA
Apparatus for cocaine SA
Rats that underwent cocaine SA were tested in operant chambers as previously described (Moretti et al, 2010). Each experimental chamber (Med Associates, St Albans, VT) was fitted with a cue light placed above each lever, and with a 2900-Hz tone module. An infusion pump was connected via an external catheter to a single-channel liquid swivel (Instech Laboratories, Plymouth Meeting, PA). Data acquisition and operant-schedule parameters were controlled by a Med-PC software (Med Associates).
Cocaine SA procedure
A total of 30 male Lister-Hooded rats (Charles-River, Margate, Kent, UK) weighing 275–300 g were individually housed in a temperature- and humidity-controlled room with water available ad libitum. Animals were food restricted throughout the experiment to maintain a constant body weight of 300 g (±10 g).
After their arrival, rats were acclimatized for 1 week and subsequently implanted with a catheter in the jugular vein as previously described (Moretti et al, 2010). After a 7-day recovery period, rats were transported to the operant chamber. Cocaine SA procedure was initiated under a fixed ratio (FR) 1 schedule of reinforcement. Each press on the active lever was associated with a 0.1 ml infusion of a cocaine hydrochloride solution (3 mg/ml, corresponding to 300 μg per infusion and 1 mg/kg in rats weighing 300 g) plus the simultaneous illumination of the stimulus (cue) light and extinction of the chamber light for 20 s. Presses on the ‘inactive’ lever had no programmed consequences. Each drug infusion (‘reward delivery’) was followed by a 20-s lever retraction. The first three ‘training’ sessions were terminated after either 50 infusions or 2 h from the start of the session. In the subsequent 30 sessions, the cocaine access time was extended to 12 h (1800–0600 h), the unit dose reduced to 0.150 μg/infusion (0.1 ml of 1.5 mg/ml cocaine solution, corresponding to 0.5 mg/kg in rats weighing 300 g), and FR gradually increased to 3 (sessions 4–6) and eventually to 5 (remaining 27 sessions).
Subjects that lost catheter patency or appeared unhealthy (ie, showed signs of infection) were removed from the study (11 subjects altogether). Repeated 48–72 h abstinence periods were introduced on days 16 (session 14, 72 h), 23 (session 18, 72 h), and 31 (session 23, 48 h) to minimize the risk of acute cocaine-induced intoxication. Session 30 was followed by a longer (5 days) binge abstinence followed by two additional sessions. Such intervals were introduced because of the necessity to harmonize the timing of MRI scan and SA protocol over the relatively large number of subjects employed. A 10-day detoxification period within the home cage was introduced before the imaging experiment.
Vehicle SA procedure
A group of 14 rats was used as baseline reference group. The subjects were implanted with a jugular catheter and subjected to the same training and SA procedures (including number, duration of SA sessions, and abstinence) as described above, except for the use of vehicle (saline, 0.1 ml) instead of cocaine during operant sessions.
Magnetic Resonance Imaging
Animal preparation
Imaging studies were performed 10 days after the last SA session. Animal preparation and MRI acquisition parameters have been previously described in greater detail (Gozzi et al, 2010; Schwarz et al, 2004). Briefly, rats were anesthetized with 3% halothane, tracheotomized, and artificially ventilated with a mechanical respirator. The femoral artery and vein were cannulated and the animals were paralyzed with D-tubocurarine. After surgery, halothane level was set to 0.8%. The body temperature of all subjects was maintained within physiological range and mean arterial blood pressure (MABP) was monitored continually through the femoral artery.
MR image acquisition
Anatomical and fMRI time series were acquired on a Bruker Avance 4.7 Tesla system. The animals were positioned prone in a custom-made holding support, and a ‘Rat Brain’ curved quadrature two-loop receive coil (Bruker, Ettlingen, Germany) was mounted on top of the animal skull and fixed to the animal holder. The animal holder was then fitted into a 72 mm birdcage resonator (Bruker) that was used for radiofrequency transmit only. Both coils are standard components provided by the manufacturer.
A T2-weighted anatomical volume was acquired using the RARE sequence (TR=5461 ms, TEeff=72 ms, RARE factor 8, FOV 40 mm, 256 × 256 matrix, 20 contiguous 1 mm slices) followed by a time-series acquisition (TReff=2700 ms, TEeff=111 ms, RARE factor 32, d.t.=27) with same spatial coverage, yielding a functional pixel volume of ≈1 mm3. Total MRI time-series acquisition time was 58 min (128 repetitions) for both groups.
Following five reference images, 2.67 ml/kg of the contrast agent Endorem (Guerbet, Roissy CdG Cedex, France) was injected to make the fMRI signal changes sensitive to cerebral blood volume (rCBV) (Mandeville et al, 1998; Schwarz et al, 2003). D-amphetamine (0.5 mg/kg) was administered intravenously 25 min after contrast agent injection, and MRI data were acquired over a period of 25 min following the challenge. The dose of D-amphetamine was chosen based on previous in vivo studies (Schwarz et al, 2004; Gozzi et al, 2011). The dose ensures robust brain activation, does not produce ‘ceiling’ rCBV responses (Micheli et al, 2007), and elicits transient MABP responses that are homeostatically compensated under halothane anesthesia (Gozzi et al, 2007; Zaharchuk et al, 1999).
Data Analysis
Basal CBV
The bCBV time-series image data for each experiment were analyzed within the framework of the general linear model (Worsley et al, 1992). Individual subjects were spatially normalized to a stereotaxic rat brain MRI template set (Schwarz et al, 2006a). Signal intensity changes were converted into bCBV(t) on a pixel-wise basis as previously described (Chen et al, 2001; Mandeville et al, 1998). bCBV time series were calculated over a 4.5-min time window starting 6.8 min after contrast agent injection. Mean bCBV volumes for individual subjects were created by averaging the 10 time points time-wise. Linear detrending was introduced to account for contrast agent washout (Schwarz et al, 2003). Voxel-wise group statistics was carried out using FSL (Smith et al, 2004) using multilevel Bayesian inference, with 0.7 mm spatial smoothing, a Z threshold >1.6, and a corrected cluster significance threshold of p=0.01.
phMRI response to D-amphetamine
MRI signal intensity changes were converted into fractional CBV (rCBV) as previously described (Mandeville et al, 1998) and detrended to account for contrast agent elimination from the blood pool (Schwarz et al, 2003). Unsmoothed, rCBV time series for amphetamine challenge were calculated covering 12.5-min prechallenge and 24-min postchallenge window. Voxel-wise statistics was carried out using FEAT with 0.7 mm spatial smoothing and using a model function (Supplementary Figure S1) capturing the temporal profile of amphetamine-induced rCBV response (Schwarz et al, 2006b). Higher-level group comparisons were carried out with multilevel Bayesian inference and thresholded at Z>1.6 with a corrected cluster significance threshold of p=0.01. In order to specifically test the hypothesis of an altered striatal reactivity to D-amphetamine in cocaine rats, a 3D binary mask of major subcortical area (striatum, thalamus, hippocampus, hypothalamus the striatum, ventral pallidum, BNST, and amygdala) was generated using a digital reconstruction of the rat brain atlas (Schwarz et al, 2006a) and used to prethreshold rCBV time series before higher-level FSL analysis. This procedure increases the statistical power of the analysis by reducing the number of multiple comparisons (Huettel et al, 2004). To investigate the regional specificity of the effect in a hypothesis-free manner and rule out generalized reductions in amphetamine response throughout the brain, the same analysis was repeated on non-masked rCBV data sets (Supplementary Figure S5). Volume of interest (VOI) mean bCBV values and time courses for the amphetamine challenge were extracted as previously described (Schwarz et al, 2006a; Gozzi et al, 2008). Statistical differences in mean bCBV were assessed using a one-way ANOVA test followed by Fisher's test for multiple comparisons.
Correlation analysis
Maps of correlated bCBV and D-amphetamine-induced rCBV responses across subjects were calculated within the GLM framework at the group level with reference to bCBV in representative regions using FSL (Schwarz et al, 2007a, 2007b). A number of representative VOIs were selected based on the results of the intergroup bCBV maps (medial prefrontal, insular, orbitofrontal, somatosensory cortex, caudate putamen, nucleus accumbens, reticular thalamus, and posteroventral thalamus). For each VOI, the design matrix comprised a regressor capturing the group mean bCBV signal in the anatomical structure and another containing the zero-mean bCBV vector across the N subjects in the group from the selected reference structure. The Z-statistic images were calculated via contrasts capturing positive and negative correlations with the reference response, and were thresholded with Z>1.6 and a corrected cluster significance threshold of p=0.01. Linear regression plots of correlated bCBV and rCBV responses were calculated by plotting bCBV and mean rCBV response to amphetamine across individual subjects, the latter being expressed as mean response over a 20 min (4–24 min postinjection) time window.
Histopathology
Histopathological evaluation was performed on 10 cocaine subject and 8 randomly chosen controls as previously described (Barroso-Moguel et al, 2002). After the MRI experiment, rats were maintained under deep anesthesia (halothane 5%), and a 15-min aortic perfusion of fixative media (10% buffered formalin) was performed, preceded by a 5-min infusion of saline. Perfused brains were removed and stored in fixative solution for further 24–72 h. Brain trimming was then performed using a brain matrix (ASI Instruments) designed for rats weighing 200–400 g. Tissue samples were paraffin embedded, sectioned into 5-μm-thin slices, and stained with a combination of hematoxylin–eosin and Luxol Fast Blue (Scholtz, 1977). The slice and brain regions analyzed were the cingulate and prefrontal cortex, caudate putamen, corpus callosum, hippocampus (C2), cerebellum (purkinje cells), and substantia nigra. The examination was performed by two study-blind veterinary pathologists.
RESULTS
Chronic Cocaine SA
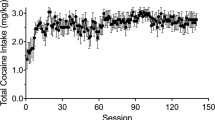
All the subjects completed the 33 cocaine SA sessions successfully over a time period of 52 days. The SA schedule used ensured a prolonged and sustained intake of cocaine throughout the study (Figure 1). The average cumulative intake of SA cocaine per subject was 1138.4 ±33.3 mg/rat. Both active level presses and cocaine intake appeared to be rather stable throughout the course of the experiment, although linear regression highlighted a weak but significant (p<0.03, F=4.62) trend toward an overall increased cocaine intake over time when all homogeneous sessions were compared (sessions 4–31, FR 3–5, binge abstinence intervals 48–72 h) (Supplementary Figure S2).
(a) Number of active lever presses recorded in the cocaine SA group (N=19) and control (saline SA, N=14) within the SA sessions. Cocaine SA procedure was initiated under a fixed ratio (FR) 1 schedule of reinforcement. The first three training sessions were terminated after either 50 infusions or 2 h from the start of the session. In the subsequent 30 sessions, the cocaine access time was extended to 12 h and FR gradually increased to 3 (sessions 4–6) and eventually to 5 (remaining 27 sessions). Repeated 48–72 h abstinence periods were introduced on days 16 (session 14, 72 h), 23 (session 18, 72 h), and 31 (session 23, 48 h) to minimize the risk of acute cocaine-induced intoxication. A 10-day detoxification period within the home cage was introduced before the imaging experiment (MRI). (b) Average cocaine intake (mg/rat/session) over the course of the experiment. The dose of cocaine administered per single injection is reported on the top line (300 μg during the training sessions 1–3, 150 μg in all remaining sessions, corresponding to 1 and 0.5 mg/kg, respectively).
Basal CBV
In order to investigate the effect of chronic cocaine administration on basal brain function, we measured bCBV in cocaine SA and control subjects and mapped the regions exhibiting statistically significant differences between groups. Rats with the SA cocaine showed significantly reduced bCBV in several brain areas compared with control rats (Figures 2 and 3). The effect was prominent in the medial-prefrontal, cingulate, orbitofrontal cortex, septum, ventral hippocampus, core region of the nucleus accumbens, as well as in raphe nuclei and reticular thalamic areas. No difference in total CBV between groups was observed (p=0.23, Student's t-test). No correlation was found between bCBV and total cocaine intake in all the VOIs examined (P>0.16, all VOIs).
Anatomical distribution of the regions exhibiting significantly lower bCBV in rats chronically self-administering cocaine (cocaine SA; N=20) vs control subjects (vehicle SA; N=14; Z>1.6, cluster correction p=0.001) in representative horizontal (a), sagittal (b), and coronal slices (c). The image planes in (a) and (b) refer to two orthogonal sections located at Ybregma −5.7 mm, and lateral (L) 2.0 mm. The rostro-caudal positions of the coronal slice boundaries in (c) are indicated in mm relative to zbregma. AcbC, core of the nucleus accumbens; Cg, cingulate cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; Plc, prelimbic cortex; Ra, raphe nucleus; rTh, reticular thalamic nucleus; Sp, septum; VHc, ventral hippocampus.
Mean bCBV in representative 3D anatomical volumes (VOIs, Schwarz et al, 2006a) for cocaine SA (N=20) and control subjects (saline SA; N=14). AcbC, core of the nucleus accumbens; AcbSh, shell of the nucleus accumbens; Amy, amygdala; Cg, cingulate cortex; Cpu, caudate putamen; Hypoth, hypothalamus; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; Ra, raphe nucleus; Rs, retrosplenial cortex; rTh, reticular thalamic nucleus; Sp, septum; SS, somatosensory cortex; VHc, ventral hippocampus. *p<0.05; **p>0.01; one-way ANOVA, followed by Fisher's LSD test.
Functional Response to D-Amphetamine
In order to probe striatal dopaminergic reactivity, cocaine SA and control rats were challenged with the DA-releaser amphetamine, and the presence of functional alterations in the magnitude of the rCBV response elicited by the drug was assessed via voxel-wise statistics. Consistent with previous studies (Schwarz et al, 2004), amphetamine produced robust activation of subcortical and cortical areas in both groups of subjects (Supplementary Figure S3). Rats chronically exposed to cocaine exhibited an attenuated functional response to amphetamine in the striatum compared with control rats (Figure 4 and Supplementary Figure S3). The effect was also apparent in un-detrended rCBV time profiles (Supplementary Figure S4). In rats that self-administered cocaine, the magnitude of the striatal response to amphetamine was found to be inversely correlated with cumulative cocaine intake (p=0.03, Figure 4). Additional foci of reduced functional response to amphetamine were observed in sensory-motor and orbitofrontal cortex (Supplementary Figures S3 and S5).
Orthogonal view (a: horizontal, b: coronal, c: sagittal) of the subcortical brain regions exhibiting an attenuated rCBV response to D-amphetamine in rats chronically self-administering cocaine (cocaine SA; N=20) vs control subjects (vehicle SA; N=14; Z>1.6; cluster correction p=0.05). The image planes in (a–c) refer to three orthogonal sections located at Ybregma (Yb) −5.2 mm, Zbregma −1.4 mm, and lateral (L) 5.2 mm, respectively. (d) Correlation of rCBV response to amphetamine in the striatum and cumulative cocaine intake over 52 days of self-administration (p=0.03, dashed lines represent 95% confidence interval). Cpu, caudate putamen.
Administration of amphetamine produced transient increases in MABP (Supplementary Figure S6). The effect was not temporally correlated with the functional response, and was well within the blood flow autoregulatory range within which vasopressive responses are homeostatically compensated without producing significant rCBV alterations (Gozzi et al, 2007; Zaharchuk et al, 1999). Arterial blood gases (paCO2 and paO2) were measured before and after the fMRI time series (Supplementary Table S1). No statistically significant difference in mean pre- or post-acquisition paCO2 values between groups was found (p>0.1, all groups; one-way ANOVA).
Correlation between Basal and Evoked Activity
In an attempt to establish a correlation between basal and evoked functional activity and to investigate dysregulations in the control of these two states, we measured the correlation between bCBV and amphetamine-induced response in control and animals that self-administered cocaine. No correlation between bCBV and amphetamine-induced rCBV responses was found in either group in any of the regions examined, with the exception of the reticular and posterior-ventral thalamus that revealed, in control subjects, an inverse relationship with amphetamine-induced rCBV in fronto-cortical areas (Supplementary Figures S7 and S8). No such correlation was present in the cocaine SA group (Supplementary Figure S8).
Histopathology
A histopathological evaluation of brain white and gray matter, glial and interstitial compartments, as well as macro- and micro-vascular, ependymal, and meningeal structures did not highlight any neurocellular, interstitial, or microvascular lesions in either group. Specifically, no signs of cellular pyknosis or atrophy, fiber alteration, necrosis, and interstitial edema were observed in any of the brain regions examined, nor alterations of the microvascular and capillary bed (ie, basal membrane dilation or rupture, hemorrhage, endothelial thickening or wall fibrosis, thrombi or occlusions, and necrosis or vacuolation of endothelial cells).
DISCUSSION
The present study documents that chronic, extended-access cocaine SA in the rat produces neuroimaging alterations that closely mimic hallmark imaging findings in human cocaine addicts. Specifically, we observed significantly reduced bCBV, a marker of resting brain function, in regions that have a key contribution in higher cognitive functions and inhibitory control (fronto-cortical areas), craving and anticipation (fronto-hippocampal areas), and reward (mesolimbic areas). Moreover, cocaine SA was associated with reduced striatal reactivity to dopaminergic stimulation, and the presence of putative functional alterations in the inhibitory interplay between reticular thalamus and the activation of fronto-cortical areas. Our results provide neuroimaging evidence of multiple alterations in rat brain function following chronic and voluntary cocaine intake that serve as plausible neurobiological substrate for the behavioral expression of compulsive drug intake in laboratory animals.
Chronic cocaine abuse is often modeled in behavioral paradigms where rodents are trained to voluntarily self-administer the drug. Here, we implemented a prolonged, extended-access cocaine SA protocol with repeated binge-abstinence periods (Parsons et al, 1995; Wilson et al, 1994; Wilson and Kish, 1996) to mimic the characteristics of high-dose, chronic cocaine abuse in humans. Prolonged SA paradigms have been reported to reproduce key clinical features of cocaine addiction including compulsive drug use despite the presence of environmental adversities (Vanderschuren and Everitt, 2004), and high propensity to relapse to drug seeking (Deroche-Gamonet et al, 2004). The chronic protocol employed (covering ≈10% of the adult lifespan of a rat, Sharp and La Regina, 1998) permits to mimic patient populations with a significant history (>6 months) of cocaine addiction like those typically enrolled in human neuroimaging studies, thus maximizing the translational relevance of our findings. Moreover, the use of extended access to cocaine (ie, ⩾6 h) is known to specifically model specific neurobehavioral features of addiction, such as persistent alterations in cognitive functions (Briand et al, 2008; George et al, 2007), increased motivation for cocaine (Paterson and Markou, 2003), and escalation in drug intake (Ahmed and Koob, 1998). Repeated periods of forced abstinence were introduced to reduce the acute toxic effects of the drug and to ensure sustained motivation to self-administer high doses of cocaine (Roberts et al, 2007). Although the total cocaine intake achieved with the present protocol is higher than that observed with short-access paradigms, the values attained are sufficiently distant from the limit of acute toxicity (Mantsch et al, 2004; Wee et al, 2007), which explains the lack of lethality observed in this study.
Compared with unlimited access protocols, where drug intake exhibits high and low numbers of infusions on alternating days (Wilson et al, 1994), the extended-access protocol used here ensured sustained SA of high doses of cocaine. In contrast to what was reported by other groups (Ahmed and Koob, 1998; Ferrario et al, 2005; Wee et al, 2007), we did not observe unequivocal evidence of dose escalation, although a trend toward an increased cocaine intake over consecutive sessions was apparent (Supplementary Figure S2).
One limitation of the model employed is that it did not include behavioral measurements of drug use despite adverse consequences (eg, ‘resistance to punishment’ Deroche-Gamonet et al, 2004), a behavioral trait that is considered an essential diagnostic criteria of addiction in humans (American Psychiatric Association, 2008). Because this feature is present in ca. 20% of rats exposed to cocaine (Deroche-Gamonet et al, 2004; Ahmed, 2010), the imaging alterations mapped in the present work are likely to include contributions from subsets of subjects exhibiting this behavior. However, whether this trait is characterized by specific functional alterations separate from those highlighted in this study remains to be determined.
A 10-day washout period was introduced before the imaging study to rule out acute carryover effects of cocaine and minimize the potential interference of acute abstinence symptoms on the measures of brain function. Most of the neurochemical and behavioral alterations that can be related to acute withdrawal have an almost immediate onset, peak between 6 and 72 h after termination of drug access, and generally cease within 2–7 days from the last cocaine session (Baumann and Rothman, 1998; Harris and Aston-Jones, 1993; Malin et al, 2000; Mutschler and Miczek, 1998; Markou and Koob, 1992). It is therefore unlikely that the imaging findings contain major perturbations from transient neurobiological phenomena related to acute cocaine abstinence. On the other hand, the observed functional alterations are expected to contain contributions from longer-lasting neuroadaptational processes (ie, incubation of cocaine craving) that have been shown to build-up after cocaine discontinuation (Lu et al, 2004), and that are of translational relevance as they can be related to propensity to relapse.
MRI measures of bCBV allow high-resolution mapping of resting brain function that tightly correlates with regional energy metabolism and cerebral blood flow (Gaisler-Salomon et al, 2009; Hyder et al, 2001; Gonzalez et al, 1995). Our data showed the presence of reduced bCBV in the cingulated gyrus, prefrontal cortex, orbitofrontal cortex, as well as in striatal and hippocampal areas of cocaine SA subjects. The frontostriatal effect is in excellent agreement with clinical neuroimaging research of cocaine addiction, where reduced frontal and striatal activities have been consistently observed (Strickland et al, 1993; Tumeh et al, 1990; London et al, 1999; Volkow et al, 1992, 1988) and found to correlate with the cognitive impairments, compulsion, and loss of inhibitory control over drug taking that may lead to relapse (Goldstein et al, 2010; Kalivas et al, 2005; Kalivas, 2004; Hong et al, 2010; Strickland et al, 1993). Importantly, cognitive deficits have been observed in rats allowed extended (but not limited) access to cocaine (Briand et al, 2008; George et al, 2007), a phenomenon that involved working memory and sustained attention tasks (two prefrontal cortex-dependent tasks) as well as object recognition measures (a hippocampus-dependent task). The involvement of hippocampal systems is also consistent with the role played by this brain structure in contextual conditioning and memory, two functions that are altered by cocaine use and are believed to play a role in cue-elicited craving (reviewed by Koob and Volkow, 2010). Likewise, the reduced bCBV in the nucleus accumbens was not unexpected, given the established interconnection between fronto-cortical activity and ventrostriatal DA cell firing and release (Kalivas et al, 2005; Peoples et al, 2007). In keeping with this, recent PET imaging studies showed lower levels of endogenous DA in cocaine addicts relative to comparison subjects (Martinez et al, 2009) and primate research revealed reduced glucose utilization in the striatal areas upon chronic cocaine use, a feature that became more pronounced with increased cocaine exposure (Porrino et al, 2007).
Focal bCBV reductions were also observed in reticular thalamic and raphe nuclei. The former finding is consistent with human neuroimaging studies showing altered GABAergic neurotransmission in the thalamus of abstinent cocaine abusers (Volkow et al, 1998) and recent electrophysiological evidence of a state of protracted overinhibition of reticular thalamic areas following binge administration of cocaine (Urbano et al, 2009). Interestingly, as serotonin exerts a direct excitatory action on GABAergic neurons in the reticular thalamus (McCormick and Wang, 1991), the reduced activity of these nuclei and that observed in regions of the raphe may be functionally interrelated and part of a single defective circuit.
No correlation between total cocaine intake and bCBV was found in any of the VOIs examined. The lack of correlation could reflect different individual susceptibility to the effect of the drug, or could be related to the high amount of cocaine self-administered that might exceed the quantity required to produce maximal bCBV alterations.
In an attempt to identify an fMRI correlate of the decreased striatal dopaminergic responsivity observed in human PET studies (Volkow et al, 1990, 1993; Martinez et al, 2004), we also mapped the functional response elicited by the DA-releaser amphetamine using a phMRI protocol (Schwarz et al, 2004; Bifone and Gozzi, 2010). Several phMRI studies have provided compelling evidence that the striatal hemodynamic response produced by amphetamine reflects primarily the dopaminergic effects (reviewed in Knutson and Gibbs, 2007). For example, amphetamine has been shown to elicit BOLD or rCBV increases in DA-rich ventrostriatal areas that are linearly correlated to synaptic DA concentrations (Dixon et al, 2005; Ren et al, 2009; Choi et al, 2006; Schwarz et al, 2007b; Preece et al, 2007). Moreover, amphetamine-induced rCBV responses are abolished in the DA denervated areas (Chen et al, 1997, 1999), an effect that can be later restored after fetal or stem cell transplantation (Bjorklund et al, 2002; Chen et al, 1999). Thus, the sum of these data indicates that amphetamine-induced rCBV responses can be reliably used as a marker of striatal DA neurotransmission. Within this framework, the presence of an attenuated striatal rCBV response to amphetamine in the cocaine SA group points toward a reduced responsivity of ventrostriatal dopaminergic function analogous to what was observed in PET studies in humans (Narendran and Martinez, 2008). This finding provides for the first time a plausible preclinical neuroimaging correlate of one of the most replicated clinical manifestation of cocaine addiction, which is believed to play a key contribution to the ‘hypohedonia’ and amotivation reported by drug-addicted subjects during protracted withdrawal (Volkow et al, 1997). This result documents a potentially important correspondence between clinical and preclinical neuroadaptational changes induced by cocaine on DA systems, an aspect that does not appear to be adequately modeled by traditional cocaine exposure paradigms, where ‘sensitized’ (ie, increased) dopaminergic responses are typically observed (reviewed by Narendran and Martinez, 2008). As similarly attenuated striatal responses were not observed in rodent neuroimaging studies using short-term (5 days) drug administration protocols (Febo et al, 2005; Reese et al, 2004; and A Gozzi, unpublished results), our data suggest that, for this characteristic to be modeled in rodents, prolonged and extended access to high doses of cocaine may be required. Importantly, no appreciable microscopic lesions in the vascular, neurocellular, and interstitial compartments of brains exposed to cocaine were observed. This result is important, as it permits to rule out a potential contribution of abnormal cerebrovascular processes on the hemodynamic measures of brain function performed (ie, bCBV and rCBV).
Correlation analysis between resting and amphetamine-evoked (rCBV) responses revealed an inverse relationship between bCBV in reticular thalamic areas and amphetamine-induced frontal activation in control subjects, but not in cocaine group. Previous studies have demonstrated that inhibition of reticular thalamic activity can enhance fronto-cortical dopaminergic neurotransmission (Jones et al, 1988), a finding consistent with the functional connectivity of these regions (Paxinos, 2008) and the high GABAergic density of reticular thalamic nucleus (Paxinos, 2008). As prefrontal projections to the thalamic reticular nucleus play a unique circuit for attentional mechanisms (Zikopoulos and Barbas, 2006), we hypothesize that the loss of correlation between basal and evoked function observed in the cocaine SA group may be related to the attentional deficits observed in rats allowed extended access to cocaine (Briand et al, 2008; George et al, 2007). A putative role for thalamo-frontal dysfunctions in cocaine addiction is supported by recent neuroimaging studies showing altered thalamo-cortical connectivity in cocaine abusers under resting conditions (Gu et al, 2010) and when performing a cognitive task (Tomasi et al, 2007). However, as correlation measurements do not reflect causal association, further research is warranted to elucidate the exact nature of this finding.
In summary, we provide evidence of altered brain function in rats that underwent prolonged and extended-access cocaine SA. Consistent with clinical neuroimaging findings, cocaine-exposed animals revealed reduced basal brain function in fronto-cortical and thalamic areas, and attenuated responsivity in striatal regions upon challenge with the DA-releaser amphetamine, an effect that was significantly correlated with the total cocaine intake. The consistency of these findings with neuroimaging measures in cocaine-addicted patients supports the use of prolonged and extended-access SA paradigms in the rat to investigate the neuroadaptations underlying cocaine addiction.
References
Ahmed SH (2010). Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev 35: 172–184.
Ahmed SH, Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300.
American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders (4th edn, revised). American Psychiatric Association: Washington, DC.
Barroso-Moguel R, Mendez-Armenta M, Villeda-Hernandez J, Nava-Ruiz C, Santamaria A (2002). Brain lesions induced by chronic cocaine administration to rats. Prog Neuropsychopharmacol Biol Psychiatry 26: 59–63.
Baumann MH, Rothman RB (1998). Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry 44: 578–591.
Bifone A, Gozzi A (2010). Functional and pharmacological MRI in understanding brain function In: Hagan J (ed). Molecular and Functional Models in Neuropsychiatry. Springer.
Bjorklund LM, Saínchez-Pernaute R, Chung S, Andersson T, Chen IYC, McNaught KS et al. (2002). Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 99: 2344–2349.
Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M et al. (2008). Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 33: 2969–2980.
Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O et al. (1997). Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med 38: 389–398.
Chen Y-CI, Brownell A-L, Galpern W, Isacson O, Bogdanov M, Beal MF et al. (1999). Detection of dopaminergic cell loss and neural transplantation using pharmacological MRI, PET and behavioural assessment. NeuroReport 10: 2881–2886.
Chen Y-CI, Mandeville JB, Nguyen TV, Talele A, Cavagna F, Jenkins BG (2001). Improved mapping of pharmacologically induced neuronal activation using the IRON technique with superparamagnetic blood pool agents. J Magn Reson Imaging 14: 517–524.
Choi JK, Chen YI, Hamel E, Jenkins BG (2006). Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage 30: 700–712.
Deroche-Gamonet V, Belin D, Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science 305: 1014–1017.
Dixon AL, Prior M, Morris PM, Shah YB, Joseph MH, Young AMJ (2005). Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology 48: 236–245.
Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF (2005). The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology 30: 936–943.
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE (2005). Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58: 751–759.
Gaisler-Salomon I, Schobel SA, Small SA, Rayport S (2009). How high-resolution basal-state functional imaging can guide the development of new pharmacotherapies for schizophrenia. Schizophr Bull 35: 1037–1044.
Gawin FH, Ellinwood EH (1988). Cocaine and other stimulants. N Engl J Med 318: 1173–1182.
George O, Mandyam CD, Wee S, Koob GF (2007). Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33: 2474–2482.
Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J et al. (2010). Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci USA 107: 16667–16672.
Gonzalez RG, Fischman AJ, Guimaraes AR, Carr CA, Stern CE, Halpern EF et al. (1995). Functional MR in the evaluation of dementia: correlation of abnormal dynamic cerebral blood volume measurements with changes in cerebral metabolism on positron emission tomography with fludeoxyglucose F 18. AJNR Am J Neuroradiol 16: 1763–1770.
Gozzi A, Ceolin L, Schwarz A, Reese T, Bertani S, Bifone A (2007). A multimodality investigation of cerebral haemodynamics and autoregulation in phMRI. Magn Reson Imaging 25: 826–833.
Gozzi A, Crestan V, Turrini G, Clemens M, Bifone A (2010). Antagonism at serotonin 5HT2a receptors modulates functional activity of fronto-hippocampal circuit. Psychopharmacology 209: 37–50.
Gozzi A, Large C, Schwarz A, Bertani S, Crestan V, Bifone A (2008). Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology 33: 1690–1703.
Gozzi A, Massagrande M, Amantini D, Antolini M, Martinelli P, Cesari N et al. (2011). Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS ONE 6: e16406.
Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA et al. (2010). Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53: 593–601.
Harris G, Aston-Jones G (1993). Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine-and morphine-dependent rats. Psychopharmacology 113: 131–136.
Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B et al. (2010). A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA 107: 13509–13514.
Huettel S, Song AW, McCarthy G (2004). Functional Magnetic Resonance Imaging. Sinauer: Sunderland.
Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL (2001). Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed 14: 413–431.
Jones MW, Kilpatrick IC, Phillipson OT (1988). Dopamine function in the prefrontal cortex of the rat is sensitive to a reduction of tonic GABA-mediated inhibition in the thalamic mediodorsal nucleus. Exp Brain Res 69: 623–634.
Kalivas PW (2004). Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4: 23–29.
Kalivas PW, Volkow N, Seamans J (2005). Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45: 647–650.
Knutson B, Gibbs S (2007). Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology 191: 813–822.
Koob GF, Sanna PP, Bloom FE (1998). Neuroscience of addiction. Neuron 21: 467–476.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
London ED, Bonson KR, Ernst M, Grant S (1999). Brain imaging studies of cocaine abuse: implications for medication development. Crit Rev Neurobiol 13: 227–242.
Lu L, Grimm JW, Hope BT, Shaham Y (2004). Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47: 214–226.
Malin DH, Moon WD, Moy ET, Jennings RE, Moy DM, Warner RL et al. (2000). A rodent model of cocaine abstinence syndrome. Pharmacol Biochem Behav 66: 323–328.
Mandeville JB, Marota JJA, Kosofsky BE, Keltner JR, Weissleder R, Rosen B et al. (1998). Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med 39: 615–624.
Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ (2004). Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology 175: 26–36.
Markou A, Koob GF (1992). Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology 7: 213–224.
Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y et al. (2004). Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29: 1190–1202.
Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R et al. (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D2/D3 receptors following acute dopamine depletion. Am J Psychiatry 166: 1170–1177.
McCormick DA, Wang Z (1991). Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol 442: 235–255.
Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A et al. (2007). 1,2,4-triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. J Med Chem 50: 5076–5089.
Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I et al. (2010). A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol Pharmacol 78: 287–296.
Mutschler NH, Miczek KA (1998). Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology 136: 402–408.
Narendran R, Martinez D (2008). Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse 62: 851–869.
Parsons LH, Koob GF, Weiss F (1995). Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther 274: 1182–1191.
Paterson NE, Markou A (2003). Increased motivation for self-administered cocaine after escalated cocaine intake. NeuroReport 14: 2229–2232.
Paxinos G (2008). The Rat Nervous System. Elsevier: London, 1193pp.
Peoples LL, Kravitz AV, Guillem K (2007). The role of accumbal hypoactivity in cocaine addiction. ScientificWorldJournal 7: 22–45.
Porrino LJ, Smith HR, Nader MA, Beveridge TJR (2007). The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry 31: 1593–1600.
Preece MA, Sibson NR, Raley JM, Blamire A, Styles P, Sharp T (2007). Region-specific effects of a tyrosine-free amino acid mixture on amphetamine-induced changes in BOLD fMRI signal in the rat brain. Synapse 61: 925–932.
Reese T, Schwarz AJ, Gozzi A, Crestan V, Bertani S, Heidbreder CA (2004). Functional magnetic resonance imaging detects spatio-temporal differences between drug-naive and amphetamine-sensitised rats. Proceedings of the Twelfth ISMRM Scientific Meeting and Exhibition. ISMRM Press: Kyoto, 228 pp.
Ren J, Xu H, Choi JK, Jenkins BG, Chen YI (2009). Dopaminergic response to graded dopamine concentration elicited by four amphetamine doses. Synapse 63: 764–772.
Roberts DCS, Morgan D, Liu Y (2007). How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry 31: 1614–1624.
Scholtz CL (1977). Quantitative histochemistry of myelin using Luxol Fast Blue MBS. Histochem J 9: 759–765.
Schwarz A, Gozzi A, Reese T, Bertani S, Crestan V, Hagan J et al. (2004). Selective dopamine D(3) receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse 54: 1–10.
Schwarz AJ, Danckaert A, Reese T, Gozzi A, Paxinos G, Watson C et al. (2006a). A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: application to pharmacological MRI. Neuroimage 32: 538–550.
Schwarz AJ, Gozzi A, Reese T, Bifone A (2007a). Functional connectivity in the pharmacologically activated brain: resolving networks of correlated responses to d-amphetamine. Magn Reson Med 57: 704–713.
Schwarz AJ, Gozzi A, Reese T, Bifone A (2007b). In vivo mapping of functional connectivity in neurotransmitter systems using pharmacological MRI. Neuroimage 34: 1627–1636.
Schwarz AJ, Reese T, Gozzi A, Bifone A (2003). Functional MRI using intravascular contrast agents: detrending of the relative cerebrovascular (rCBV) time course. Magn Reson Imaging 21: 1191–1200.
Schwarz AJ, Whitcher B, Gozzi A, Reese T, Bifone A (2006b). Study-level wavelet cluster analysis and data-driven signal models in pharmacological MRI. J Neurosci Methods 159: 346–360.
Sharp PM, La Regina MC (1998). The Laboratory Rat. CRC Press: Berlin, 240 pp.
Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA (2004). Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA 101: 7181–7186.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1): S208–S219.
Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM et al. (1993). Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci 5: 419–427.
Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC et al. (2007). Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res 155: 189–201.
Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N et al. (2010). Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE 5: e10815.
Tumeh SS, Nagel JS, English RJ, Moore M, Holman BL (1990). Cerebral abnormalities in cocaine abusers: demonstration by SPECT perfusion brain scintigraphy. Work in progress. Radiology 176: 821–824.
Urbano FJ, Bisagno Vn, Wikinski SI, Uchitel OD, Llin RR (2009). Cocaine acute ‘binge’ administration results in altered thalamocortical interactions in mice. Biol Psychiatry 66: 769–776.
Vanderschuren LJMJ, Everitt BJ (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–1019.
Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ et al. (1993). Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14: 169–177.
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64: 1575–1579.
Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R et al. (1990). Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147: 719–724.
Volkow ND, Hitzemann RJ, Wang GJ, Fowler JS, Wolf AP, Dewey SL et al. (1992). Long-term frontal brain metabolic changes in cocaine abusers. Synapse 12: 86.
Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K (1988). Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry 152: 641–648.
Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Gatley SJ, Dewey SS et al. (1998). Enhanced sensitivity to benzodiazepines in active cocaine-abusing subjects: a PET Study. Am J Psychiatry 155: 200–206.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al. (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386: 830–833.
Wee S, Specio SE, Koob GF (2007). Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther 320: 1134–1143.
Wilson JM, Kish SJ (1996). The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci 16: 3507–3510.
Wilson JM, Nobrega JN, Carroll ME, Niznik HB, Shannak K, Lac ST et al. (1994). Heterogeneous subregional binding patterns of 3H-WIN 35,428 and 3H-GBR 12,935 are differentially regulated by chronic cocaine self- administration. J Neurosci 14: 2966–2979.
Worsley KJ, Evans AC, Marrett S, Neelin P (1992). A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918.
Zaharchuk G, Mandeville JB, Bogdanov Jr AA, Weissleder R, Rosen BR, Marota JJ (1999). Cerebrovascular dynamics of autoregulation and hypoperfusion. An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke 30: 2197–2204.
Zikopoulos B, Barbas H (2006). Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci 26: 7348–7361.
Acknowledgements
We thank Valerio Crestan and Giuliano Turrini for their excellent technical support to the phMRI measures, and Pamela Rodegher from Histolab, Verona, Italy, for the histological preparations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All the authors are employees of GlaxoSmithKline. The authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Gozzi, A., Tessari, M., Dacome, L. et al. Neuroimaging Evidence of Altered Fronto-Cortical and Striatal Function after Prolonged Cocaine Self-Administration in the Rat. Neuropsychopharmacol 36, 2431–2440 (2011). https://doi.org/10.1038/npp.2011.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.129
Keywords
This article is cited by
-
A sex-dependent role for the prelimbic cortex in impulsive action both before and following early cocaine abstinence
Neuropsychopharmacology (2021)
-
Risky decision-making in individuals with substance use disorder: A meta-analysis and meta-regression review
Psychopharmacology (2020)
-
Dorsolateral striatal miR-134 modulates excessive methamphetamine intake in self-administering rats
Metabolic Brain Disease (2019)
-
Dopamine and addiction: what have we learned from 40 years of research
Journal of Neural Transmission (2019)
-
Longitudinal Changes in Brain Metabolic Activity after Withdrawal from Escalation of Cocaine Self-Administration
Neuropsychopharmacology (2017)