Abstract
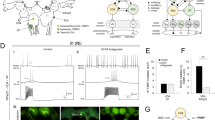
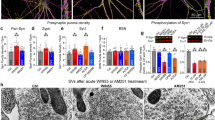
The brain's endocannabinoid retrograde messenger system decreases presynaptic transmitter release, but its physiological function is uncertain. We show that endocannabinoid signaling is absent when spatially dispersed synapses are activated on rodent cerebellar Purkinje cells but that it reduces presynaptic glutamate release when nearby synapses are active. This switching of signaling according to the spatial pattern of activity is controlled by postsynaptic type I metabotropic glutamate receptors, which are activated disproportionately when glutamate spillover between synapses produces synaptic crosstalk. When spatially distributed synapses are activated, endocannabinoid inhibition of transmitter release can be rescued by inhibiting glutamate uptake to increase glutamate spillover. Endocannabinoid signaling initiated by type I metabotropic glutamate receptors is a homeostatic mechanism that detects synaptic crosstalk and downregulates glutamate release in order to promote synaptic independence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marr, D. A theory of cerebellar cortex. J. Physiol. (Lond.) 202, 437–470 (1969).
Ito, M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol. Rev. 81, 1143–1195 (2001).
Barbour, B. & Hausser, M. Intersynaptic diffusion of transmitter. Trends Neurosci. 20, 377–384 (1997).
Marcaggi, P., Billups, D. & Attwell, D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fiber synapses. J. Physiol. (Lond.) 552, 89–107 (2003).
Barbour, B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron 11, 759–769 (1993).
Wilson, R.I. & Nicoll, R.A. Endocannabinoid signaling in the brain. Science 296, 678–682 (2002).
Kreitzer, A.C. & Regehr, W.G. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 12, 324–330 (2002).
Coutinho, V., Mutoh, H. & Knöpfel, T. Functional topology of the mossy fiber-granule cell-Purkinje cell system revealed by imaging of intrinsic fluorescence in mouse cerebellum. Eur. J. Neurosci. 20, 740–748 (2004).
Batchelor, A.M. & Garthwaite, J. Novel synaptic potentials in cerebellar Purkinje cells: probable mediation by metabotropic glutamate receptors. Neuropharmacology 32, 11–20 (1993).
Tempia, F., Miniaci, M.C., Anchisi, D. & Strata, P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J. Neurophysiol. 80, 520–528 (1998).
Brown, S.P., Brenowitz, S.D. & Regehr, W.G. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat. Neurosci. 6, 1048–1057 (2003).
Levenes, C., Daniel, H. & Crepel, F. Retrograde modulation of transmitter release by postsynaptic subtype 1 metabotropic glutamate receptors in the rat cerebellum. J. Physiol. (Lond.) 537, 125–140 (2001).
Kreitzer, A.C. & Regehr, W. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29, 717–727 (2001).
Maejima, T., Hashimoto, K., Yoshida, T., Alba, A. & Kano, M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31, 463–475 (2001).
Brenowitz, S.D. & Regehr, W.G. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron 45, 419–431 (2005).
Eccles, J.C., Llinas, R. & Sasaki, K. The mossy fiber-granule cell relay in the cerebellum and its inhibition by Golgi cells. Exp. Brain Res. 1, 82–101 (1966).
Kim, S.J., Kim, Y.S., Yuan, J.P., Petralia, R.S., Worley, P.F. & Linden, D.J. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426, 285–291 (2003).
Takechi, H., Eilers, J. & Konnerth, A. A new class of synaptic response involving calcium release in dendritic spines. Nature 396, 757–760 (1998).
Batchelor, A.M. & Garthwaite, J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature 385, 74–77 (1997).
Kushmerick, C. et al. Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J. Neurosci. 24, 5955–5965 (2004).
Brasnjo, G. & Otis, T.S. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron 31, 607–616 (2001).
Diana, M.A., Levenes, C., Mackie, K. & Marty, A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J. Neurosci. 22, 200–208 (2002).
Brenowitz, S.D. & Regehr, W.G. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J. Neurosci. 23, 6373–6384 (2003).
Sims, R.E. & Hartell, N.A. Differences in transmission properties and susceptibility to long-term depression reveal functional specialization of ascending axon and parallel fiber synapses to Purkinje cells. J. Neurosci. 25, 3246–3257 (2005).
Neale, S.A., Garthwaite, J. & Batchelor, A.M. Metabotropic glutamate receptor subtypes modulating neurotransmission at parallel fiber-Purkinje cell synapses in rat cerebellum. Neuropharmacology 41, 42–49 (2001).
Acknowledgements
We thank K. Tanaka for providing the knockout mice, and B. Barbour, A. Gibb, D. Rossi, A. Silver and M. Hamann for comments on the manuscript. Supported by the European Union, the Wellcome Trust and a Wolfson-Royal Society award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Marcaggi, P., Attwell, D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci 8, 776–781 (2005). https://doi.org/10.1038/nn1458
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1458
This article is cited by
-
Developmental timing-dependent organization of synaptic connections between mossy fibers and granule cells in the cerebellum
Communications Biology (2023)
-
Evaluation, simulation, and optimization of land use spatial patterns for high-quality development: A case study of Zhengzhou city, China
Journal of Geographical Sciences (2023)
-
Activation of the mGlu1 metabotropic glutamate receptor has antipsychotic-like effects and is required for efficacy of M4 muscarinic receptor allosteric modulators
Molecular Psychiatry (2020)
-
Is Purkinje Neuron Hyperpolarisation Important for Cerebellar Synaptic Plasticity? A Retrospective and Prospective Analysis
The Cerebellum (2020)
-
Coordinated regulation of endocannabinoid-mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase
Scientific Reports (2016)