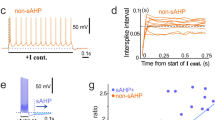
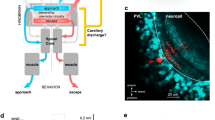
Abstract
The axon initial segment (AIS) serves as the site of action potential initiation in most neurons, but difficulties in isolating the effects of voltage-gated ion channels in the AIS from those of the soma and dendrites have hampered understanding how AIS properties influence neural coding. Here we have combined confocal microscopy, patch-clamp recordings and light-sensitive channel blockers ('photoswitches') in binaural auditory gerbil neurons to show that hyperpolarization and cyclic-nucleotide-gated (HCN) channels are expressed in the AIS and decrease spike probability, in a manner distinct from that of HCN channels in the soma and dendrites. Furthermore, the control of spike threshold by HCN channels in the AIS can be altered through serotonergic modulation of 5-hydroxytryptamine 1A (5-HT1A) receptors, which hyperpolarizes the activation range of HCN channels. As release of serotonin signals changes in motivation and attention states, axonal HCN channels provide a mechanism to translate these signals into changes in the threshold for sensory stimuli.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Coombs, J.S., Curtis, D.R. & Eccles, J.C. The interpretation of spike potentials of motoneurones. J. Physiol. (Lond.) 139, 198–231 (1957).
Stuart, G.J. & Sakmann, B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367, 69–72 (1994).
Grubb, M.S. et al. Short- and long-term plasticity at the axon initial segment. J. Neurosci. 31, 16049–16055 (2011).
Yoshimura, T. & Rasband, M.N. Axon initial segments: diverse and dynamic neuronal compartments. Curr. Opin. Neurobiol. 27, 96–102 (2014).
Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007).
Kuba, H., Ishii, T.M. & Ohmori, H. Axonal site of spike initiation enhances auditory coincidence detection. Nature 444, 1069–1072 (2006).
Hu, W. et al. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 12, 996–1002 (2009).
Kole, M.H., Letzkus, J.J. & Stuart, G.J. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55, 633–647 (2007).
Grubb, M.S. & Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074 (2010).
Kuba, H., Oichi, Y. & Ohmori, H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 465, 1075–1078 (2010).
Bender, K.J. & Trussell, L.O. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 61, 259–271 (2009).
Bender, K.J., Ford, C.P. & Trussell, L.O. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron 68, 500–511 (2010).
Martinello, K. et al. Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron 85, 346–363 (2015).
Grothe, B. New roles for synaptic inhibition in sound localization. Nat. Rev. Neurosci. 4, 540–550 (2003).
Berghs, S. et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J. Cell Biol. 151, 985–1002 (2000).
Van Wart, A., Trimmer, J.S. & Matthews, G. Polarized distribution of ion channels within microdomains of the axon initial segment. J. Comp. Neurol. 500, 339–352 (2007).
Mourot, A., Tochitsky, I. & Kramer, R.H. Light at the end of the channel: optical manipulation of intrinsic neuronal excitability with chemical photoswitches. Front. Mol. Neurosci. 6, 5 (2013).
Hurley, L.M. & Thompson, A.M. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. J. Comp. Neurol. 435, 78–88 (2001).
Thompson, A.M. & Hurley, L.M. Dense serotonergic innervation of principal nuclei of the superior olivary complex in mouse. Neurosci. Lett. 356, 179–182 (2004).
Lehnert, S. et al. Action potential generation in an anatomically constrained model of medial superior olive axons. J. Neurosci. 34, 5370–5384 (2014).
Scott, L.L., Mathews, P.J. & Golding, N.L. Perisomatic voltage-gated sodium channels actively maintain linear synaptic integration in principal neurons of the medial superior olive. J. Neurosci. 30, 2039–2050 (2010).
Khurana, S. et al. An essential role for modulation of hyperpolarization-activated current in the development of binaural temporal precision. J. Neurosci. 32, 2814–2823 (2012).
Koch, U., Braun, M., Kapfer, C. & Grothe, B. Distribution of HCN1 and HCN2 in rat auditory brainstem nuclei. Eur. J. Neurosci. 20, 79–91 (2004).
Kopp-Scheinpflug, C., Pigott, B.M. & Forsythe, I.D. Nitric oxide selectively suppresses IH currents mediated by HCN1-containing channels. J. Physiol. (Lond.) 593, 1685–1700 (2015).
Santoro, B. et al. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 20, 5264–5275 (2000).
Wainger, B.J., DeGennaro, M., Santoro, B., Siegelbaum, S.A. & Tibbs, G.R. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411, 805–810 (2001).
Ishii, T.M., Takano, M., Xie, L.-H., Noma, A. & Ohmori, H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J. Biol. Chem. 274, 12835–12839 (1999).
Bucchi, A., Tognati, A., Milanesi, R., Baruscotti, M. & DiFrancesco, D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J. Physiol. (Lond.) 572, 335–346 (2006).
Kramer, R.H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
Mathews, P.J., Jercog, P.E., Rinzel, J., Scott, L.L. & Golding, N.L. Control of submillisecond synaptic timing in binaural coincidence detectors by K(v)1 channels. Nat. Neurosci. 13, 601–609 (2010).
Baumann, V.J., Lehnert, S., Leibold, C. & Koch, U. Tonotopic organization of the hyperpolarization-activated current (Ih) in the mammalian medial superior olive. Front. Neural Circuits 7, 117 (2013).
Harnett, M.T., Magee, J.C. & Williams, S.R. Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J. Neurosci. 35, 1024–1037 (2015).
Magee, J.C. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 2, 848 (1999).
Poolos, N.P., Migliore, M. & Johnston, D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat. Neurosci. 5, 767–774 (2002).
McCormick, D.A. & Pape, H.C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. (Lond.) 431, 291–318 (1990).
Narayanan, R. & Johnston, D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 56, 1061–1075 (2007).
Golding, N.L., Robertson, D. & Oertel, D. Recordings from slices indicate that octopus cells of the cochlear nucleus detect coincident firing of auditory nerve fibers with temporal precision. J. Neurosci. 15, 3138–3153 (1995).
Dodson, P.D., Barker, M.C. & Forsythe, I.D. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J. Neurosci. 22, 6953–6961 (2002).
Colbert, C.M. & Pan, E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 5, 533–538 (2002).
Steinbusch, H.W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6, 557–618 (1981).
Polter, A.M. & Li, X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell. Signal. 22, 1406–1412 (2010).
Wang, J., Chen, S., Nolan, M.F. & Siegelbaum, S.A. Activity-dependent regulation of HCN pacemaker channels by cyclic AMP: signaling through dynamic allosteric coupling. Neuron 36, 451–461 (2002).
Cotel, F., Exley, R., Cragg, S.J. & Perrier, J.F. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc. Natl. Acad. Sci. USA 110, 4774–4779 (2013).
Yin, L. et al. Selective modulation of axonal sodium channel subtypes by 5-HT1A receptor in cortical pyramidal neuron. Cereb. Cortex http://dx.doi.org/10.1093/cercor/bhv245 (2015).
Tang, Z.Q. & Trussell, L.O. Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J. Neurosci. 35, 4540–4551 (2015).
Sturgill, J.F. & Isaacson, J.S. Somatostatin cells regulate sensory response fidelity via subtractive inhibition in olfactory cortex. Nat. Neurosci. 18, 531–535 (2015).
Wilson, N.R., Runyan, C.A., Wang, F.L. & Sur, M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348 (2012).
Yamada, R., Kuba, H., Ishii, T.M. & Ohmori, H. Hyperpolarization-activated cyclic nucleotide-gated cation channels regulate auditory coincidence detection in nucleus laminaris of the chick. J. Neurosci. 25, 8867–8877 (2005).
Franken, T.P., Roberts, M.T., Wei, L., Golding, N.L. & Joris, P.X. In vivo coincidence detection in mammalian sound localization generates phase delays. Nat. Neurosci. 18, 444–452 (2015).
Roberts, M.T., Seeman, S.C. & Golding, N.L. A mechanistic understanding of the role of feedforward inhibition in the mammalian sound localization circuitry. Neuron 78, 923–935 (2013).
Chang, K.J. et al. Glial ankyrins facilitate paranodal axoglial junction assembly. Nat. Neurosci. 17, 1673–1681 (2014).
Rasband, M.N. et al. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 19, 7516–7528 (1999).
Yang, Y., Lacas-Gervais, S., Morest, D.K., Solimena, M. & Rasband, M.N. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J. Neurosci. 24, 7230–7240 (2004).
Schafer, D.P., Custer, A.W., Shrager, P. & Rasband, M.N. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol. 2, 69–79 (2006).
Scott, L.L., Mathews, P.J. & Golding, N.L. Posthearing developmental refinement of temporal processing in principal neurons of the medial superior olive. J. Neurosci. 25, 7887–7895 (2005).
Banghart, M.R. et al. Photochromic blockers of voltage-gated potassium channels. Angew. Chem. Int. Edn. Engl. 48, 9097–9101 (2009).
Tochitsky, I. et al. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81, 800–813 (2014).
Acknowledgements
The authors would like to acknowledge S. Khurana, who first observed that 5-HT activates 5-HT3 receptors in MSO neurons. This work was supported by NIH grants NS044916 (M.N.R.), EY024334 (R.H.K.) and DC006877 (N.L.G.).
Author information
Authors and Affiliations
Contributions
K.W.K. performed and analyzed all experiments in brain slices. M.N.R. performed and analyzed immunofluorescence labeling experiments. V.M. performed and analyzed experiments in HEK cells. R.H.K. and N.L.G. supervised and helped design experiments in expression systems and slices, respectively. K.W.K. and N.L.G. wrote the main manuscript text with assistance from M.N.R. and R.H.K. M.N.R. prepared Figure 1, K.W.K. prepared Figures 2, 3, 4, 5, 6, 7, 8 and Supplementary Figures 2, 3, 4, 5, 6, 7, and V.M. and R.H.K. prepared Supplementary Figure 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 DENAQ enables photo-control of HCN channels expressed in HEK‑293 cells.
Whole-cell recordings from a HEK-293 cell co-transfected with the mouse HCN4 gene and the fluorescent marker mCherry. The pipette solution included 200 µM DENAQ. (a) HCN currents were activated in dark (black) and in 488 nm light (green) by a series of incremental hyperpolarizing voltage steps from −40 mV to −100 mV. The duration of each step was shortened to better reveal the kinetics of HCN current activation. (b) HCN current was activated by a single hyperpolarizing step from −40 mV to −120 mV. A 1 second flash of 480 nm relieved blockade of the channels and increased the current. External Cs+, which blocks HCN channels, reversibly eliminated the light-dependent increase. In four experiments, the mean increase in current in 480 nm light was 44 ± 9% (mean±SEM).
Supplementary Figure 2 Decrease in spike probability and changes in spike shape from somatic HCN channel block arise from postsynaptic membrane hyperpolarization
(a) Stimulus protocol (top) and example responses (bottom) to a train of 10 synaptic stimuli delivered at 100 Hz. A glass pipette (~50 µm diameter) was used to stimulate contralateral glutamatergic inputs while glycinergic inhibitory inputs were blocked by bath application of 1 µM strychnine. Stimulus amplitude was adjusted to trigger ~50% suprathreshold responses. AAQ treated cells were field illuminated at 380 nm (left), after which the soma was scanned at 488 nm (center), hyperpolarizing the cell. Synaptic responses were again elicited after the resting potential was restored to control values with direct somatic current injection (right) (b) Phase plane analysis of the first action potential triggered in the train show that voltage threshold and spike shape are not altered with HCN channel block in with somatic 488 nm illumination. Dotted lines: spike threshold. (c-f). Group data showing that the decrease in spike probability is eliminated when the resting potential is restored to control values during somatic scanning at 488 nm (p = 0.02 and 0.56 for soma and soma + DC; n=4). Spike shape parameters are unchanged (soma and soma+DC respectively: Vrest, p=0.01 and 0.4; Max dV/dt, p=0.19 and 0.76; spike threshold, p=0.73 and 0.19; n=4). One-way repeated measures ANOVA. Error bars indicate SEM.
Supplementary Figure 3 Strong hyperpolarization during HCN blockade mimics the effects of light-induced HCN block in MSO neurons.
(a) Experimental diagram showing that direct current (DC) is injected through a recording pipette to hyperpolarize resting membrane potentials. Synaptic stimuli were evoked in neurons recorded with internal 20 µM ZD7288. (b) Averaged action potential under control condition (black trace) and during −8 mV membrane hyperpolarization (red trace). (c) Phase plane plots for the records in b. (d‑f) Group data quantifying average changes in action potential probability, threshold and maximum dV/dt as a function of somatic hyperpolarization (spike probability: 2 mV, p=0.004; 4 mV, p=0.037; 6 mV, p=0.002; 8 mV, p=0.009; 10 mV, p=0.0008; threshold: 2 mV, p=0.07; 4 mV, p=0.08; 6 mV, p=0.008; 8 mV, p=0.0007; 10 mV, p=0.03); Max. dV/dt: 2 mV, p=0.06; 4 mV, p=0.12; 6 mV, p=0.003; 8 mV, p=0.0001; 10 mV, p=0.02). One-way repeated measures ANOVA. Error bars indicate SEM. * p<0.05; *** p<0.001.
Supplementary Figure 4 Voltage-dependent unblock of Ih in MSO neurons in the presence of ivabradine.
(a) Relief of blockade of Ih by 10 µM ivabradine during prolonged hyperpolarization. Schematic and left traces: hyperpolarizing/depolarizing voltage prepulses from –100 to +5 mV, followed by a step to –100 mV for 40 s. (b) Expanded view of prepulses. (c) Group data of showing Ih amplitude during the 1st (a) and 5th (b) prepulses, as well as the end of the test pulse (c).
Supplementary Figure 5 Time course of serotonin modulation of Ih.
(a) Voltage dependence of Ih activation in whole-cell voltage-clamp recordings (voltage steps: −30 mV to −110 mV in −10 mV steps, 1 s duration). Decreased Ih after bath application of 20 µM 5-HT shows extensive recovery after washout (black traces). b. Time course of Ih during 5-HT application (red). Instantaneous leak current remains relatively stable (gray). (a), (b), and (c) indicate time points of example traces in a. (c) Time course of changes in half-activation voltage of Ih. (d) Normalized conductance plots of V1/2 for the time points shown in b and c, while instantaneous leak current (gray) is constant. (e) Quantification of group data for the change in maximum current and V1/2 for Ih in 20 µM 5-HT and washout conditions relative to control (red bars). The amplitude of Ih but not instantaneous leak current differs significantly from the 5-HT condition (current amplitude: Ih, p = 0.029, instantaneous leak, p=0.73; Ih V1/2, p=0.017 (n=3). 2-tailed paired t test. Error bars indicate SEM. * p<0.05
Supplementary Figure 6 Changes in spike probability and spike shape from photoswitch block of the AIS or soma are eliminated in the presence of a blocker of HCN channels.
(a) MSO cells were recorded with 20 µM ZD7288 included in the pipette solution to block HCN channels internally. Stimulus protocol (top) and example responses (bottom) to a train of 10 synaptic stimuli delivered at 100 Hz. A glass pipette (~50 µm diameter) was used to stimulate contralateral glutamatergic inputs while glycinergic inhibitory inputs were blocked by bath application of 1 µM strychnine. Stimulus amplitude was adjusted to trigger ~50% suprathreshold responses. AAQ treated cells were field illuminated at 380 nm (left), after which either the AIS or soma was scanned at 488 nm (center and right, respectively). (b) Phase plane analysis of the first action potential triggered in the train comparing control conditions (at 380 nm) vs. 488 nm scanning of the AIS (left) and soma (right). No changes are apparent in spike shape or threshold (dotted lines) with HCN channel block. (c-f) Group data showing that there are no significant changes in spike probability and other electrophysiological parameters at either the AIS or soma in recordings with internal ZD7288 (spike probability: AIS, p = 0.88; Soma, p = 0.56; resting potential: AIS, p = 0.08; Soma, p = 0.98; spike threshold: AIS, p = 0.91; Soma, p = 0.22; maximum dV/dt: AIS, p = 0.18; Soma, p = 0.83; n=5. One-way repeated measures ANOVA. Error bars indicate SEM.
Supplementary Figure 7 Spatial resolution of 488 nm confocal laser scanning of the AIS in MSO cells pre-incubated in 300 µM AAQ.
(a) AIS of an MSO principal neuron filled with 40 µM Alexa Fluor 568 hydrazide. A series of four 30 µm long regions of interest (yellow box) were drawn, first over the AIS, and then laterally offset from the AIS in 0.5 µm increments (only the two extreme positions of the ROIs are shown for clarity). (b) Cells were held constantly in 380 nm field illumination to maintain AAQ in the unblocked configuration. Each ROI was then scanned at 488 nm, and the change in resting potential was measured. Cells were returned to the 380 nm condition after each scan. The change in membrane potential fell exponentially with distance from the AIS and exhibited a distance constant of 0.37 µm, demonstrating that the compartment-specific effects of AAQ blockade are minimally affected by light scattering in the slice. Scale bar, 10 µm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 926 kb)
Rights and permissions
About this article
Cite this article
Ko, K., Rasband, M., Meseguer, V. et al. Serotonin modulates spike probability in the axon initial segment through HCN channels. Nat Neurosci 19, 826–834 (2016). https://doi.org/10.1038/nn.4293
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4293
This article is cited by
-
Regulation of Axon Initial Segment Diameter by COUP-TFI Fine-tunes Action Potential Generation
Neuroscience Bulletin (2022)
-
An axon-specific expression of HCN channels catalyzes fast action potential signaling in GABAergic interneurons
Nature Communications (2020)
-
Optical control of neuronal ion channels and receptors
Nature Reviews Neuroscience (2019)