Abstract
The cerebral cortex is plastic and represents the world according to the significance of sensory stimuli. However, cortical networks are embodied in complex circuits, including neuromodulatory systems such as the noradrenergic locus coeruleus, providing information about internal state and behavioral relevance. Although norepinephrine is important for cortical plasticity, it is unknown how modulatory neurons themselves respond to changes of sensory input. We examined how locus coeruleus neurons are modified by experience and the consequences of locus coeruleus plasticity for cortical representations and sensory perception. We made whole-cell recordings from rat locus coeruleus and primary auditory cortex (A1), pairing sounds with locus coeruleus activation. Although initially unresponsive, locus coeruleus neurons developed and maintained auditory responses afterwards. Locus coeruleus plasticity induced changes in A1 responses lasting at least hours and improved auditory perception for days to weeks. Our results demonstrate that locus coeruleus is highly plastic, leading to substantial changes in regulation of brain state by norepinephrine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Katz, L.C. & Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Hensch, T.K. Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004).
Feldman, D.E. & Brecht, M. Map plasticity in somatosensory cortex. Science 310, 810–815 (2005).
Gilbert, C.D., Li, W. & Piech, V. Perceptual learning and adult cortical plasticity. J. Physiol. (Lond.) 587, 2743–2751 (2009).
Levelt, C.N. & Hübener, M. Critical-period plasticity in the visual cortex. Annu. Rev. Neurosci. 35, 309–330 (2012).
Znamenskiy, P. & Zador, A.M. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013).
Buonomano, D.V. & Merzenich, M.M. Cortical plasticity: from synapses to maps. Annu. Rev. Neurosci. 21, 149–186 (1998).
Fritz, J. et al. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat. Neurosci. 6, 1216–1223 (2003).
Ohl, F.W. & Scheich, H. Learning-induced plasticity in animal and human auditory cortex. Curr. Opin. Neurobiol. 15, 470–477 (2005).
Froemke, R.C. & Martins, A.R.O. Spectrotemporal dynamics of auditory cortical synaptic receptive field plasticity. Hear. Res. 279, 149–161 (2011).
Sanes, D.H. & Woolley, S.M. A behavioral framework to guide research on central auditory development and plasticity. Neuron 72, 912–929 (2011).
Marlin, B.J., Mitre, M., D'amour, J.A., Chao, M.V. & Froemke, R.C. Oxytocin enables maternal behavior by balancing cortical inhibition. Nature 520, 499–504 (2015).
Bakin, J.S. & Weinberger, N.M. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc. Natl. Acad. Sci. USA 93, 11219–11224 (1996).
Marder, E. From biophysics to models of network function. Annu. Rev. Neurosci. 21, 25–45 (1998).
Shulz, D.E. et al. A neuronal analogue of state-dependent learning. Nature 403, 549–553 (2000).
Gu, Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835 (2002).
Froemke, R.C. et al. A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007).
Constantinople, C.M. & Bruno, R.M. Effects and mechanisms of wakefulness on local cortical networks. Neuron 69, 1061–1068 (2011).
Chun, S. et al. Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J. Neurosci. 33, 7345–7357 (2013).
Bear, M.F. & Singer, W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176 (1986).
Hu, H. et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131, 160–173 (2007).
Bush, D.E. et al. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front. Behav. Neurosci. 4, 154 (2010).
Edeline, J.M. et al. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear. Res. 274, 75–84 (2011).
Kuo, S.P. & Trussell, L.O. Spontaneous spiking and synaptic depression underlie noradrenergic control of feed-forward inhibition. Neuron 71, 306–318 (2011).
Eldar, E. et al. The effects of neural gain on attention and learning. Nat. Neurosci. 16, 1146–1153 (2013).
Aston-Jones, G. & Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Berridge, C.W. Noradrenergic modulation of arousal. Brain Res. Rev. 58, 1–17 (2008).
Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223 (2009).
Carter, M.E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Aston-Jones, G. et al. Locus coeruleus neurons are selectively activated by attended cues in a vigilance task. J. Neurosci. 14, 4467–4480 (1994).
Usher, M. et al. The role of locus coeruleus in the regulation of cognitive performance. Science 283, 549–554 (1999).
Yu, A.J. & Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005).
Sugiyama, D. et al. In vivo patch-clamp recording from locus coeruleus neurones in the rat brainstem. J. Physiol. (Lond.) 590, 2225–2231 (2012).
Devilbiss, D.M. & Waterhouse, B.D. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J. Neurophysiol. 105, 69–87 (2011).
Froemke, R.C. et al. Long-term modification of cortical synapses improves sensory perception. Nat. Neurosci. 16, 79–88 (2013).
Sara, S.J. & Segal, M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog. Brain Res. 88, 571–585 (1991).
Aston-Jones, G., Rajkowski, J., Kubiak, P. & Alexinsky, T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 14, 4467–4480 (1994).
Johansen, J.P. et al. Molecular mechanisms of fear learning and memory. Cell 147, 509–524 (2011).
Disney, A.A. & Reynolds, J.H. Expression of m1-type muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: a comparative study of rat, guinea pig, ferret, macaque, and human. J. Comp. Neurol. 522, 986–1003 (2014).
Muñoz, W. & Rudy, B. Spatiotemporal specificity in cholinergic control of neocortical function. Curr. Opin. Neurobiol. 26, 149–160 (2014).
Froemke, R.C. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci. 38, 195–219 (2015).
Pawlak, V., Greenberg, D.S., Sprekeler, H., Gerstner, W. & Kerr, J.N. Changing the responses of cortical neurons from sub- to suprathreshold using single spikes in vivo. eLife 2, e00012 (2013).
Bao, S., Chan, V.T. & Merzenich, M.M. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 412, 79–83 (2001).
Bieszczad, K.M. & Weinberger, N.M. Representational gain in cortical area underlies increase of memory strength. Proc. Natl. Acad. Sci. USA 107, 3793–3798 (2010).
Schultz, W., Dayan, P. & Montague, P.R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Bromberg-Martin, E.S., Matsumoto, M. & Hikosaka, O. Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron 67, 144–155 (2010).
Shumake, J., Ilango, A., Scheich, H., Wetzel, W. & Ohl, F.W. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J. Neurosci. 30, 5876–5883 (2010).
Seamans, J.K. & Yang, C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 74, 1–58 (2004).
Huang, Y., Mylius, J., Scheich, H. & Brosch, M. Tonic effects of the dopaminergic ventral midbrain on the auditory cortex of awake macaque monkeys. Brain Struct. Funct. (in the press) (2014).
Gunaydin, L.A. et al. Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392 (2010).
Acknowledgements
We thank I. Carcea, C. Clopath, A.M. Gomez, M. Jin, J.E. LeDoux, M.A. Long, L.J. Miller, C.N. Parkhurst, D.B. Polley, S.D. Shea, I. Shehu and N. Zaika for comments, discussions and technical assistance, and C.A. Loomis and the New York University School of Medicine Histology Core for assistance with anatomical studies. J. Pivkova created artwork in Figures 1a and 2a. S.E. Ross created artwork in Supplementary Figure 12a. This work was funded by NIDCD (DC009635 and DC012557), a Hirschl/Weill-Caulier Career Research Award and a Sloan Research Fellowship (R.C.F.), and the Portuguese Foundation for Science and Technology (A.R.O.M.).
Author information
Authors and Affiliations
Contributions
A.R.O.M. performed the experiments. A.R.O.M. and R.C.F. analyzed the experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Recording from locus coeruleus.
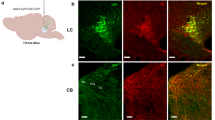
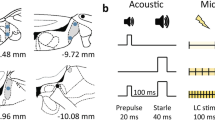
a, Noxious stimuli evoke phasic spike bursts in rat locus coeruleus. Top, brief tail pinch (100 msec duration) evoked phasic spiking in locus coeruleus, as measured through the stimulation electrode. Bottom, foot shock (100 Hz for 500 msec, 50 V) also evoked high frequency bursts of activity as measured with unit recordings. Traces shown are from separate animals. b, Pairing pure tones with foot shock leads to a long-lasting increase in tone-evoked spiking in locus coeruleus. Top, example traces showing that before foot shock pairing ('Pre'), presentation of a pure tone (8 kHz, 80 dB SPL, 50 msec duration) did not evoke significant spiking in locus coeruleus. Ten minutes after pairing foot shock (as in a) with 8 kHz tones, the paired tone by itself evoked spiking responses in locus coeruleus. Bottom, post-stimulus time histogram of multiunit recording from locus coeruleus showing change in firing rate after tonal presentation just before (gray) and one hour after (red) pairing. Pairing induced a large (~1 Hz) increase in firing rate with ~30 msec latency. c, Locus coeruleus neuron filled with biocytin (red) during cell-attached recording in vivo and co-stained for tyrosine hydroxylase (TH, green) and DAPI (blue). Biocytin filled cells were recovered in two animals. Scale bars: 400 μm (tissue); 100 msec and 5 mV (recording). d, Whole-cell current-clamp recording from locus coeruleus in vivo. Shown are a cluster of co-labeled biocytin-positive and TH-positive neurons; four consecutive recordings were made in this animal from the same location, but we cannot determine which recordings came from which neurons or whether some biocytin reactivity is due to diffusion (perhaps by gap junctions) or leakage from the whole-cell pipette. Biocytin filled cells were recovered in two animals. Scale bar: 50 μm; (tissue); 200 msec and 40 mV (recording). e, Post hoc identification of locus coeruleus electrode site in fixed tissue after electrolytic lesion (30 s, 0.4 mA). Electrode tip was first dipped in DiI (red); tissue co-stained for DAPI (blue), tyrosine hydroxylase (TH). Electrode location was assessed this way in two animals. Scale bar: 400 μm. f, Local field potentials (LFPs) measured at distances from locus coeruleus stimulation electrode (20 Hz for 500 msec, 20 V). LFPs were only observed <500 μm, within the spatial extent of locus coeruleus. Error bars show s.e.m.
Supplementary Figure 2 Comparison of locus coeruleus activity under two different anesthetics (ketamine/dexemedetomidine vs. pentobarbital).
a, Multiunit recording of ongoing activity in locus coeruleus. Top, example traces. Bottom, summary of activity before (open bars) and after pairing (filled bars) under ketamine/dexmedetomidine (black; before pairing: 0.8±0.5 Hz, after pairing: 0.4±0.1 Hz, N = 3 animals) or pentobarbital anesthesia (purple; before pairing: 1.6±0.4 Hz, after pairing: 0.6±0.03 Hz, N = 3, P = 0.3 for before pairing and P = 0.29 after pairing, compared to ketamine/dexmedetomidine anesthesia with Student's two-tailed unpaired t-tests). Note decrease in spontaneous activity. b, Multiunit recording of footshock-evoked activity in locus coeruleus. Top, example traces (foot shock of 50 V at 100 Hz for 500 msec; only last 200 msec of foot shock is shown). Bottom, summary of responses across a range of different shock intensities under ketamine/dexmedetomidine (black; N = 3) or pentobarbital anesthesia (purple; N = 4, P = 0.4 compared to ketamine/dexmedetomidine anesthesia with ANOVA). Error bars show s.e.m.
Supplementary Figure 3 Summary of new tonal responses in locus coeruleus neurons after pairing (non-normalized responses).
a, Synaptic responses (33 measurements, 14 neurons, eight animals; EPSP before pairing: 0.3±0.2 mV; EPSP 5-15 minutes post-pairing: 1.5±0.4 mV, P = 0.009 compared to baseline EPSP before pairing with Student's unpaired two-tailed t-test; EPSP 3-10 hours post-pairing: 1.0±0.2 mV, P = 0.04 compared to baseline). Filled diamonds, experiments using single-cell depolarization instead of extracellular stimulation ('ES') for pairing. Open symbols, AP5 in locus coeruleus (seven neurons, three animals; EPSP before pairing: 0.4±0.4 mV; EPSP 5-15 minutes post-pairing: –0.04±0.03 mV, P = 0.3; EPSP 3-10 hours post-pairing: 0.8±0.4 mV, P = 0.4). b, Spiking responses (35 measurements, 20 neurons, 10 animals; spiking before pairing: 0.2±0.2 spikes/tone; spiking 5-15 minutes post-pairing: 0.7±0.3 spikes/tone, P = 0.2; spiking 3-10 hours post-pairing: 0.8±0.2 spikes/tone, P = 0.03; AP5, 13 neurons, five animals; spiking before pairing: 0.02±0.02 spikes/tone; spiking 5-15 minutes post-pairing: 0.01±0.01 spikes/tone, P = 0.7; spiking 3-10 hours post-pairing: 0.03±0.03 spikes/tone, P = 0.8).
Supplementary Figure 4 Summary of changes to AI tuning curves after optogenetic locus coeruleus pairing.
a, Synaptic and spiking tuning curves before and 0-5 hours post-pairing from current-clamp (filled) or cell-attached recordings (open). Each recording from same AI location. Upper left, first recording ten minutes before (gray) and fifteen minutes after (black) pairing optical stimulation with 16 kHz (arrow). Arrowhead, original best frequency (1 kHz). EPSPs increased across frequencies (paired 16 kHz EPSPs: 0.2±0.03 mV pre-pairing, 4.9±1.0 mV post-pairing, P = 0.0001; unpaired EPSPs across other frequencies: 0.2±0.01 mV pre-pairing, 3.8±0.3 mV post-pairing, P = 10-13). Upper right, second cell-attached recording 150 minutes post-pairing (best frequency shift: 100%). Lower left, third recording 210 minutes post-pairing (shift: 100%). Lower right, fourth recording 270 minutes post-pairing (shift: 100%). b, Shifts in AI synaptic tuning curve peaks. After 2-8 hours post-pairing, tuning curve peak remained shifted to the paired frequency (shift: 95.8±4.2%, P = 10-5 compared to baseline tuning, six cells from four animals). Shaded area, mean± s.e.m. of shifts from Figure 4c. c, Changes in AI synaptic tuning curve width after optogenetic pairing. After 2-8 hours post-pairing, tuning curve width was not significantly different from baseline (width: 115.0±31.6%, P = 0.6). Shaded area, mean± s.e.m. of shifts from Figure 4d. d, Spiking best frequency also remained shifted to the paired input (shift after 2-8 hours: 112.5±18.0%, P = 0.002 compared to baseline tuning, six cells from three animals). Shaded area, mean±s.e.m. from Figure 4e. e, Spiking tuning curve widths recovered after 2-8 hours (width: 161.7±23.7%, P = 0.05). Shaded area, mean± s.e.m. from Figure 4f. Error bars show s.e.m.
Supplementary Figure 5 Locus coeruleus pairing modifies intensity tuning of AI neurons.
a, Example current-clamp recording showing synaptic intensity tuning (measured at 8 kHz) before and after locus coeruleus pairing at a lower intensity (50 dB SPL). b, Example current-clamp recording showing spiking intensity tuning (at 8 kHz) before and after locus coeruleus pairing at higher intensity (70 dB SPL). c, Summary of changes to evoked synaptic and spiking responses after pairing. Left, EPSPs evoked by the paired intensity before and after pairing (pre: 1.0±0.5 mV, post: 5.0±1.7 mV; n = 6, P = 0.03). Right, tone-evoked spikes before and after pairing (pre: 0.05±0.03 spikes/tone, post: 0.35±0.11 spikes/tone; n = 6, P = 0.015). Error bars show s.e.m.
Supplementary Figure 6 Extracellular recordings from AI before and after locus coeruleus pairing.
a, Example multiunit recording from adult rat AI before (gray dashed lines), 10-20 minutes after (top, black solid line) and six hours after (bottom, black solid line) pairing locus coeruleus ('LC') stimulation with 1 kHz tones (arrow). Original best frequency at this AI location was 8 kHz (open arrowhead). b, Multiunit recording from a different animal, showing spiking responses 10-20 minutes (top) and 11 hours (bottom) after pairing 60 dB SPL 2 kHz tones with locus coeruleus stimulation. Even after 11 hours, tuning curves maintained preference for the paired frequency, while general tuning structure and overall excitability had recovered. Original best frequency here was initially also 8 kHz. c, Same recording as in b, showing intensity tuning before and 6.5 hours after locus coeruleus pairing. d, Summary of long-term changes to spiking responses at paired and unpaired stimuli measured with multiunit recordings 10-20 minutes post-pairing (spiking response at paired stimulus: 374.5±53.9% of baseline spiking, n = 7, P = 0.002 compared to baseline with Student's paired two-tailed t-test; increase of spiking response at unpaired stimuli: 297.6±63.1%, P = 0.02) and ~6 hours post-pairing (paired stimulus: 389.2±94.5%, n = 5, P = 0.03; unpaired stimuli: 191.9±33.7%, P = 0.1). Error bars show s.e.m.
Supplementary Figure 7 Effects of locus coeruleus pairing under pentobarbital anesthesia.
a, Four recordings from adult rat AI 20 minutes before (upper left, gray dashed lines), 30 minutes after (upper right, black solid line), two hours after (lower left) and four hours after (lower right) pairing locus coeruleus ('LC') stimulation with 2 kHz tones (arrow). Original best frequency at this AI location was 8 kHz (open arrowhead). b, Multiunit recording from a different animal, showing spiking responses before and 4 hours after pairing. Original best frequency at this AI location was 4 kHz (open arrowhead). c, Best frequency shifts after locus coeruleus pairing under pentobarbital anesthesia (shift after 3-6 hours: 92.9±4.6%, P = 10-6 compared to baseline tuning, seven recordings from five animals). Circles, whole-cell current-clamp recordings; squares, cell-attached recordings; diamonds, extracellular recordings of multiunit activity. Shaded area, mean± s.e.m. from Figure 4e. Error bars show s.e.m.
Supplementary Figure 8 Duration of synaptic frequency tuning modifications induced by a single episode of nucleus basalis pairing.
a, Normalized frequency tuning of excitation (filled) and inhibition (open) for the first cell 20 minutes before pairing. Currents were normalized to the amplitude of the largest EPSC and IPSC across frequencies. Arrow, paired frequency (4 kHz). Arrowhead, original best frequency (1 kHz). b, Second cell from same region of AI, recorded 125 minutes after pairing. c, Third cell from same region of AI, recorded 295 minutes after pairing. d, Time course for the normalized shift in excitatory tuning curve peak; tuning returned to baseline after 4-8 hours (P = 0.3). 0% represents the original best frequency for a given AI location; 100% is a full shift to the paired frequency. Data from 52 recordings in 24 animals; 31 of these recordings were previously published in Froemke et al., Nature 2007. Time is relative to the end of pairing. Error bars show s.e.m.
Supplementary Figure 9 Extracellular recordings from AI before and after locus coeruleus pairing in the presence of atropine.
a, Example multiunit recording from adult rat AI before (gray dashed lines) and 10-20 minutes after (top, black solid line) pairing locus coeruleus ('LC') stimulation with 2 kHz tones in 1 mM atropine (arrow). Atropine was applied just prior to pairing and continuously over a six-hour duration post-pairing. Changes are similar to those observed when cholinergic transmission is intact, showing that muscarinic receptor activation is not involved during or after locus coeruleus pairing. Original best frequency at this AI location was 8 kHz (open arrowhead). b, Summary of long-term changes to spiking responses at paired and unpaired stimuli measured with multiunit recordings 10-20 minutes post-pairing (spiking response at paired stimulus: 382.5±116.8% of baseline spiking, n = 3, P = 0.6 compared to experiments without atropine from Supplementary Figure 6d with Student's unpaired two-tailed t-test; increase of spiking response at unpaired stimuli: 313.8±57.8%, P = 0.6 compared to locus coeruleus pairing effects at unpaired inputs from Supplementary Figure 6d) and 3-6 hours post-pairing (paired stimulus: 261.3±47.5%, P = 0.1; unpaired stimuli: 172.7±21.1%, P = 0.3). Error bars show s.e.m.
Supplementary Figure 10 Locus coeruleus pairing increases excitatory and inhibitory tone-evoked responses.
a, Example voltage-clamp recording of excitatory (filled symbols) and inhibitory (open symbols) synaptic frequency tuning curves from an AI neuron before (gray dashed line) and after pairing (black solid line). Arrow, paired frequency (4 kHz). EPSCs evoked by the paired frequency increased from –12.2±4.4 pA to –75.9±21.3 pA and IPSCs increased from 2.0±0.9 pA to 112.8±50.9 pA. Same recording as shown in Figure 5a. b, A second example of excitatory and inhibitory tuning curve modifications induced by locus coeruleus pairing. EPSCs evoked by the paired frequency (2 kHz) increased from –24.7± 4.3 pA to –38.6±7.6 pA and IPSCs increased from 27.7±15.5 pA to 50.5±10.7 pA. c, Time course of changes to tone-evoked IPSCs before and after pairing, for both the paired (triangles) and average of unpaired tones (upside-down triangles) for 16 recordings (same recordings as shown for excitation in Figures 4 and 5). d, Summary of changes to tone-evoked IPSCs and excitatory-inhibitory correlation after pairing. Left, voltage-clamp recordings of IPSCs evoked by paired tones before and after pairing (pre: 15.1±3.1 pA, post: 31.0±8.6 pA; n = 16, P = 0.04, Student's paired two-tailed t-test). Right, linear correlation coefficient r of excitation and inhibition for all presented tones before and after pairing was unchanged (pre: 0.5±0.1, post: 0.5±0.1 mV; n = 21, P = 0.8). Error bars show s.e.m.
Supplementary Figure 11 Long-term synaptic modifications enabled by disinhibition.
a, Pairing repetitive tonal stimulation with bicuculline iontophoresis modified synaptic tuning curves. Top and middle, example of 2 kHz tones paired with GABAA receptor antagonist bicuculline (10 mM iontophoretically applied). Note that there was no locus coeruleus stimulation here. Excitation (filled symbols) at the paired frequency was enhanced (before: –44.5±7.0 pA, after: –78.6±10.2 pA, increase of 76.6%, P = 0.008). Inhibition (open symbols) at the paired frequency was also enhanced (before: 40.1±8.7 pA, after: 109.5±26.9 pA, increase of 173.3%, P = 0.01). Lines indicate mean tuning curves before (gray dashed) and 10 minutes after (black solid) induction. Arrow indicates frequency of the paired tone. Insets, currents before and after pairing. Bottom, summary of bicuculline pairing experiments without locus coeruleus pairing. Excitation increased during and after pairing (during bicuculline pairing: increase of 31.1±11.6%, P = 0.02; 10-20 minutes after pairing: increase of 45.6±6.3%, n = 14, P = 10-4), whereas pairing transiently reduced inhibition before tone-evoked inhibitory responses were strengthened (during bicuculline pairing: decrease of 47.0±5.5%, P = 0.0003; 10-20 minutes after pairing: increase of 52.7±9.1%, P = 0.0002). Horizontal bar, duration of bicuculline pairing. b, Repetitive tonal stimulation enhanced excitation when cells were perfused with picrotoxin ('PTX'). Top and middle, example of 8 kHz tones presented with GABAA receptor antagonist picrotoxin perfused into this neuron (1 mM in whole-cell pipette). Note that there was no locus coeruleus stimulation here, nor was inhibition blocked in other neurons. Tones were not presented for the first 10 minutes after break-in (at time –10 minutes) to allow whole-cell dialysis with picrotoxin. Afterwards, excitation increased (EPSPs at 0-1 minutes: –108.4±15.4 pA, EPSCs at 9-10 minutes: –288.1±11.7 pA, increase of 165.8%, P = 10-11). Top, full duration of recording; middle, first 10 minutes of tonal stimulation. Insets, EPSCs at 0-1 and 9-10 minutes. Bottom, summary of picrotoxin experiments. In some recordings, MK-801 (1 mM) was also included in the whole-cell pipette to block NMDA receptors. Excitation increased during tonal stimulation (increase of 62.9±23.0% after 10 minutes, n = 6, P = 0.04), but not if NMDA receptors were also blocked (increase of 3.4±3.4%, n = 5, P = 0.3). Horizontal bar, single-cell application of picrotoxin with or without MK-801. Error bars show s.e.m.
Supplementary Figure 12 Frequency recognition abilities are first reduced before being enhanced by pairing.
a, Behavioral training. Adult female rats were operantly conditioned to nosepoke to a target tone (4 kHz) for a food reward; other non-target tones were unrewarded. b, Pairing initially impaired and then improved 'narrowband' performance (d' pre: 0.52, 1-2 hours post: 0.11, 12 hours post: 0.79). c, Summary of impairment to recognition 1-2 hours after pairing (d' before: 0.39±0.07, after: –0.06±0.09, N = 12, P = 10-4). AP5 infusion to locus coeruleus blocked effects of pairing (open circles; d' before: 0.22±0.04, after: 0.19±0.08, N = 6, P = 0.6). d, Summary of recognition enhancement 12 hours after pairing (d' after: 0.53±0.07, P = 0.01). Locus coeruleus infusion of AP5 prevented this (open circles; d' after: 0.21±0.06, P = 0.8). Error bars show s.e.m.
Supplementary Figure 13 Multiple episodes of nucleus basalis pairing over several days are required to persistently enhance auditory perception
a, One additional week of training on the detection task, without pairing, does not lead to long-term improvements. Animals were first trained for 1-2 weeks on the wideband task at 70 dB SPL before all frequency-intensity foils were used. Low intensity detection for this example animal was similar during days 1-2 (d' at 30 dB SPL: 0.7; dashed lines) as on days 7-8 (d' at 30 dB SPL: 0.7; solid lines), showing that perceptual improvements did not occur in absence of nucleus basalis or locus coeruleus pairing. Circles, responses to target 4 kHz tones; triangles, responses to foils. b, Enduring gains in baseline detection performance after several daily episodes of nucleus basalis pairing. Baseline (pre-pairing) behavioral responses just prior to nucleus basalis pairing from a different animal than that in a, from days 1-2 (d' at 30 dB SPL: 0.0) and days 7-8 (d' at 30 dB SPL: 1.3). Baseline performance was substantially enhanced after a week of daily, five minute episodes of nucleus basalis pairing. c, Improved baseline detection abilities required 3+ daily episodes of nucleus basalis pairing. Starting on the third day of training on the detection task, nucleus basalis stimulation was paired with low intensity (30-45 dB SPL) 4 kHz tones, once/day for roughly five minutes. Other animals received no nucleus basalis stimulation (open symbols). Daily baseline performance of unpaired animals did not improve after a week or more of daily training (Δd': –0.1±0.1, N = 9, P = 0.5), possibly due to a general failure to perceive such quiet tones and associate these subliminal stimuli with reward. Daily baseline performance of paired animals, however, gradually improved over a similar period (Δd': 0.2±0.05, N = 9, P = 0.0009), indicating that animals began to perceive these stimuli even without nucleus basalis pairing, and that this perceptual improvement lasted at least for several days. d, One week of training on the narrowband recognition task, without pairing, eventually leads to long-term improvements. Animals were first trained for 1-2 weeks on the wideband task before narrowband foils were used. Performance for this animal was lower during the first two days (d': 0.5) than on the seventh and eighth days (d': 0.7), indicating that when animals are specifically trained for a week or longer, perceptual improvements can occur for relatively loud, salient stimuli in absence of pairing. e, Nucleus basalis pairing accelerates performance improvement on narrowband recognition. Baseline (pre-pairing) behavioral responses just prior to nucleus basalis pairing from a different animal than in a, from the first and second days (d' at 30 dB SPL: 0.0) and the seventh and eighth days (d' at 30 dB SPL: 1.3). Baseline performance was substantially enhanced after a week of daily, five minute episodes of nucleus basalis pairing. f, Time course of improved baseline narrowband recognition abilities with (black symbols; Δd': 0.4±0.1, N = 7, P = 0.0009) or without (open symbols; Dd': 0.3±0.1, N = 4, P = 0.02) several days of nucleus basalis pairing. Error bars show s.e.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 2375 kb)
Rights and permissions
About this article
Cite this article
Martins, A., Froemke, R. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci 18, 1483–1492 (2015). https://doi.org/10.1038/nn.4090
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4090
This article is cited by
-
Dendrites help mitigate the plasticity-stability dilemma
Scientific Reports (2023)
-
Locus coeruleus activity improves cochlear implant performance
Nature (2023)
-
Adaptive mechanisms facilitate robust performance in noise and in reverberation in an auditory categorization model
Communications Biology (2023)
-
Neural circuit for social authentication in song learning
Nature Communications (2022)
-
Spatiotemporal dynamics of noradrenaline during learned behaviour
Nature (2022)