Abstract
The scaffolding protein ankyrin-G is required for Na+ channel clustering at axon initial segments. It is also considered essential for Na+ channel clustering at nodes of Ranvier to facilitate fast and efficient action potential propagation. However, notwithstanding these widely accepted roles, we show here that ankyrin-G is dispensable for nodal Na+ channel clustering in vivo. Unexpectedly, in the absence of ankyrin-G, erythrocyte ankyrin (ankyrin-R) and its binding partner βI spectrin substitute for and rescue nodal Na+ channel clustering. In addition, channel clustering is also rescued after loss of nodal βIV spectrin by βI spectrin and ankyrin-R. In mice lacking both ankyrin-G and ankyrin-R, Na+ channels fail to cluster at nodes. Thus, ankyrin R–βI spectrin protein complexes function as secondary reserve Na+ channel clustering machinery, and two independent ankyrin-spectrin protein complexes exist in myelinated axons to cluster Na+ channels at nodes of Ranvier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hill, A.S. et al. Ion channel clustering at the axon initial segment and node of ranvier evolved sequentially in early chordates. PLoS Genet. 4, e1000317 (2008).
Susuki, K. et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron 78, 469–482 (2013).
Zonta, B. et al. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J. Cell Biol. 181, 1169–1177 (2008).
Feinberg, K. et al. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron 65, 490–502 (2010).
Thaxton, C., Pillai, A.M., Pribisko, A.L., Dupree, J.L. & Bhat, M.A. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron 69, 244–257 (2011).
Kordeli, E., Lambert, S. & Bennett, V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J. Biol. Chem. 270, 2352–2359 (1995).
Bennett, V. & Baines, A.J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81, 1353–1392 (2001).
Yang, Y., Ogawa, Y., Hedstrom, K.L. & Rasband, M.N. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J. Cell Biol. 176, 509–519 (2007).
Gasser, A. et al. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 Na+ channels to axon initial segments and nodes of Ranvier. J. Neurosci. 32, 7232–7243 (2012).
Dzhashiashvili, Y. et al. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 177, 857–870 (2007).
Zhou, D. et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143, 1295–1304 (1998).
Pan, Z. et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 26, 2599–2613 (2006).
Hedstrom, K.L. et al. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 178, 875–886 (2007).
Lemaillet, G., Walker, B. & Lambert, S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 278, 27333–27339 (2003).
Garrido, J.J. et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300, 2091–2094 (2003).
Sherman, D.L. et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 48, 737–742 (2005).
Salzer, J.L. Polarized domains of myelinated axons. Neuron 40, 297–318 (2003).
Eshed-Eisenbach, Y. & Peles, E. The making of a node: a co-production of neurons and glia. Curr. Opin. Neurobiol. 23, 1049–1056 (2013).
Susuki, K. & Rasband, M.N. Molecular mechanisms of node of Ranvier formation. Curr. Opin. Cell Biol. 20, 616–623 (2008).
Buttermore, E.D., Thaxton, C.L. & Bhat, M.A. Organization and maintenance of molecular domains in myelinated axons. J. Neurosci. Res. 91, 603–622 (2013).
Zhou, X. et al. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc. Natl. Acad. Sci. USA 107, 9424–9429 (2010).
Furuta, Y., Lagutin, O., Hogan, B.L. & Oliver, G.C. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26, 130–132 (2000).
Chan, W., Kordeli, E. & Bennett, V. 440-kD ankyrinB: structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J. Cell Biol. 123, 1463–1473 (1993).
Lambert, S., Davis, J.Q. & Bennett, V. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J. Neurosci. 17, 7025–7036 (1997).
Galiano, M.R. et al. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell 149, 1125–1139 (2012).
Ogawa, Y. et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J. Neurosci. 26, 5230–5239 (2006).
Scotland, P., Zhou, D., Benveniste, H. & Bennett, V. Nervous system defects of AnkyrinB(−/−) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD AnkyrinB in premyelinated axons. J. Cell Biol. 143, 1305–1315 (1998).
Bennett, V. & Stenbuck, P.J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature 280, 468–473 (1979).
Lux, S.E. et al. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature 345, 736–739 (1990).
Rasband, M.N. et al. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 19, 7516–7528 (1999).
Bréchet, A. et al. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J. Cell Biol. 183, 1101–1114 (2008).
Parkinson, N.J. et al. Mutant β-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nat. Genet. 29, 61–65 (2001).
Yang, Y., Lacas-Gervais, S., Morest, D.K., Solimena, M. & Rasband, M.N. βIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J. Neurosci. 24, 7230–7240 (2004).
Susuki, K. Node of Ranvier disruption as a cause of neurological diseases. ASN Neuro 5, 209–219 (2013).
Leterrier, C., Brachet, A., Dargent, B. & Vacher, H. Determinants of voltage-gated sodium channel clustering in neurons. Semin. Cell Dev. Biol. 22, 171–177 (2011).
Barry, J. et al. Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons. Dev. Cell 28, 117–131 (2014).
Bennett, V. & Healy, J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol. Med. 14, 28–36 (2008).
Zhang, Y. et al. Assembly and maintenance of nodes of Ranvier rely on distinct sources of proteins and targeting mechanisms. Neuron 73, 92–107 (2012).
Chang, K.J. et al. Glial ankyrins facilitate paranodal axoglial junction assembly. Nat. Neurosci. 10.1038/nn.3858 (2 November 2014).
Zhang, X. & Bennett, V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J. Cell Biol. 142, 1571–1581 (1998).
Lambert, S. & Bennett, V. Postmitotic expression of ankyrinR and beta R-spectrin in discrete neuronal populations of the rat brain. J. Neurosci. 13, 3725–3735 (1993).
Kordeli, E. & Bennett, V. Distinct ankyrin isoforms at neuron cell bodies and nodes of Ranvier resolved using erythrocyte ankyrin-deficient mice. J. Cell Biol. 114, 1243–1259 (1991).
Peters, L.L. et al. Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nb mice. J. Cell Biol. 114, 1233–1241 (1991).
McCann, S.R. & Jacob, H.S. Spinal cord disease in hereditary spherocytosis: report of two cases with a hypothesized common mechanism for neurologic and red cell abnormalities. Blood 48, 259–263 (1976).
Jenkins, P.M. et al. E-cadherin polarity is determined by a multifunction motif mediating lateral membrane retention through ankyrin-G and apical-lateral transcytosis through clathrin. J. Biol. Chem. 288, 14018–14031 (2013).
Weed, S.A., Stabach, P.R., Oyer, C.E., Gallagher, P.G. & Morrow, J.S. The lethal hemolytic mutation in beta I sigma 2 spectrin Providence yields a null phenotype in neonatal skeletal muscle. Lab. Invest. 74, 1117–1129 (1996).
Berghs, S. et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J. Cell Biol. 151, 985–1002 (2000).
Auld, V.J. et al. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron 1, 449–461 (1988).
Schafer, D.P., Bansal, R., Hedstrom, K.L., Pfeiffer, S.E. & Rasband, M.N. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J. Neurosci. 24, 3176–3185 (2004).
Zhang, C., Susuki, K., Zollinger, D.R., Dupree, J.L. & Rasband, M.N. Membrane domain organization of myelinated axons requires betaII spectrin. J. Cell Biol. 203, 437–443 (2013).
Radonić, A. et al. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 313, 856–862 (2004).
Acknowledgements
We thank K. Susuki for discussions. This research was supported by US National Institutes of Health grants NS044916 (MNR), NS069688 (MNR), NS49119 (ECC), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and CURE (Citizens United for Research on Epilepsy). V.B. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
M.N.R. and T.S.-Y.H. conceived the project, designed the experiments and wrote the manuscript. D.R.Z. performed the electrophysiology experiments and analyzed the data. M.N.R. performed intravitreal injections of AAV. T.S.-Y.H. performed all other experiments and analyzed the data. K.-J.C. supervised the RT-qPCR experiments. K.-J.C., M.X., E.C.C., M.C.S. and V.B. provided crucial reagents, mice and support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Na+ channel clustering is delayed in AnkG-deficient axons.
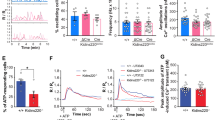
(a) P0 Ank3F/F, Nestin-Cre; Ank3F/+, and Nestin-Cre; Ank3F/F mouse brain cortices stained with antibodies against AnkG (green) and Na+ channels (red). Scale bar, 10 µm. (b) Comparison between Ank3F/F (N=7) and Avil-Cre; Ank3F/F (N= 8) mice using the wire-hang test. All mice successfully stayed on the wire for the 60 second duration of the test. (c) Comparison between Ank3F/F (N=8) and Avil-Cre; Ank3F/F (N=8) mice using the hot-plate assay to test for thermal nociception (p=0.27, unpaired two-tailed t-test, error bars indicate +/– SEM). (d) Representative recordings of compound action potentials from P14 dorsal roots from Ank3F/F and Avil-Cre; Ank3F/F mice. (e) The ratio of the intensity for Na+ channel immunostaining to AnkB immunostaining in Ank3F/F (N=27 nodes) and Avil-Cre; Ank3F/F (N= 36 nodes) dorsal roots at P7. Error bars indicate +/– SEM. (f) Immunostaining of nodes of Ranvier from dorsal roots of P7 Ank3F/F and Avil-Cre; Ank3F/F mice using antibodies against Na+ channels (red) and AnkB (green). Scale bar, 10 µm.
Supplementary Figure 2 AnkG-deficient nodes of Ranvier remain intact in aged mice.
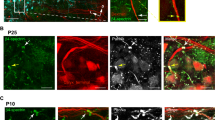
(a,b) Ank3F/F and Avil-Cre; Ank3F/F dorsal roots (a), and Ank3F/F and Six3-Cre; Ank3F/F optic nerve (b) immunostained using antibodies against Na+ channels (red), Caspr (blue), and AnkG (green). Nodes (arrows) of AnkG-deficient axons have Na+ channels. (c,d) Ank3F/F and Avil-Cre; Ank3F/F dorsal roots (c), and Ank3F/F and Six3-Cre; Ank3F/F optic nerve (d) immunostained using antibodies against Na+ channels (red), neurofascin (NF)/Caspr (blue), and AnkR (green). Nodes (arrows) of AnkG-deficient axons have high densities of AnkR colocalized with Na+ channels. Scale bars, 10 µm.
Supplementary Figure 3 AnkG-deficient nodes of Ranvier have Na+ channels that co-localize with AnkR and βI spectrin.
(a,b) Ank3F/F and Avil-Cre; Ank3F/F dorsal roots (a), and Ank3F/F and Six3-Cre; Ank3F/F optic nerve (b) immunostained using antibodies against Na+ channels (red), neurofascin (NF)/Caspr (blue), and AnkR (green). Nodes of AnkG-deficient axons have high densities of Na+ channels colocalized with AnkR. (c,d) Ank3F/F and Avil-Cre; Ank3F/F dorsal roots (c), and Ank3F/F and Six3-Cre; Ank3F/F optic nerve (d) immunostained using antibodies against Na+ channels (red), Caspr (blue), and βI spectrin (green). Some nodes (arrows) of AnkG-deficient axons have Na+ channels colocalized with βI spectrin. Scale bars, 10 µm.
Supplementary Figure 4 βIV spectrin is lost from many AnkG-deficient nodes.
(a,b) Ank3F/F (a) and Avil-Cre; Ank3F/F (b) dorsal roots immunostained using antibodies against AnkB (red), βIV spectrin (blue), and AnkR (green). Nodes (arrow) of AnkG-deficient axons have little or no βIV spectrin. Scale bar, 10 µm.
Supplementary Figure 5 AnkR is not detected at developing optic nerve nodes of Ranvier.
(a) Immunoblots of dorsal roots from postnatal day 14 and 4 months using antibodies against AnkR and neurofilament-m (NF-M) as a loading control. (b) Immunostaining of wild-type optic nerves using antibodies against Caspr (red), βIV spectrin (blue), and AnkR (green) at postnatal days 10, 21, and 30. Scale bar, 10 µm.
Supplementary Figure 6 Schematic representation of the mechanisms of sodium channel clustering at nodes of Ranvier.
(a) Cartoons illustrate the substitution of AnkR and βI spectrin results in the compensation for loss of AnkG and βIV spectrin in Avil-Cre; Ank3F/F, and the absence of Na+ channel clustering in AnkG/AnkR-deficient axons despite intact paranodal junctions. (b) Model illustrating the primary and secondary ankyrin/spectrin-dependent clustering mechanisms found at nodes of Ranvier. Higher affinities among nodal proteins are indicated by solid lines, while lower-affinity interactions are indicated by dashed lines.
Supplementary Figure 7 Na+ channels are clustered at nodes of Ranvier in exon 1b AnkG knockout (KO) mice.
(a, b) nodes of Ranvier labeled for Caspr (red), AnkG (blue), and Nav1.6 Na+ channels (green) in cerebellum of P90 WT (a) and exon 1b AnkG KO mice (b). Scale bar, 10 µm.
Supplementary Figure 8 Axon initial segments fail to form in AnkG-deficient retinal and dorsal root ganglion cells.
(a) Immunostaining of retinas from Ank3F/F and Six3-Cre;Ank3F/F mice using antibodies against AnkG (green), Na+ channels (red), and AnkR (blue). In the Ank3F/F retina the axon initial segment is indicated by the arrowheads (left panels). In the Six3-Cre;Ank3F/F retina the axon, with diffuse AnkR but no AnkG clustering, is indicated by arrowheads (right panels). The retinal ganglion cell is indicated by an asterisk. (b) Immunostaining of dorsal root ganglia from Ank3F/F and Avil-Cre;Ank3F/F mice using antibodies against AnkG (green), Na+ channels (red), and AnkR (blue). In the Ank3F/F dorsal root ganglion the axon initial segment is indicated by the arrowheads and a node is indicated by the arrow (left panels). The Avil-Cre;Ank3F/F dorsal root ganglia have no axon initial segments although nodes of Ranvier are intact and labeled with AnkR antibodies (arrows; right panels). Scale bars, 10 µm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 3740 kb)
Supplementary Methods Checklist
(PDF 396 kb)
Rights and permissions
About this article
Cite this article
Ho, TY., Zollinger, D., Chang, KJ. et al. A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat Neurosci 17, 1664–1672 (2014). https://doi.org/10.1038/nn.3859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3859
This article is cited by
-
The T-type calcium channelosome
Pflügers Archiv - European Journal of Physiology (2024)
-
Hierarchical graph learning for protein–protein interaction
Nature Communications (2023)
-
Impairment of μ-calpain activation by rhTNFR:Fc reduces severe burn-induced membrane disruption in the heart
Cell Death Discovery (2022)
-
Loss of β4-spectrin impairs Nav channel clustering at the heminode and temporal fidelity of presynaptic spikes in developing auditory brain
Scientific Reports (2022)
-
Mechanisms of node of Ranvier assembly
Nature Reviews Neuroscience (2021)