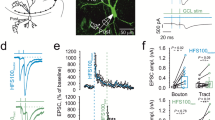
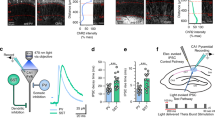
Abstract
NMDA receptors (NMDARs) are classically known as coincidence detectors for the induction of long-term synaptic plasticity and have been implicated in hippocampal CA3 cell–dependent spatial memory functions that likely rely on dynamic cellular ensemble encoding of space. The unique functional properties of both NMDARs and mossy fiber projections to CA3 pyramidal cells place mossy fiber NMDARs in a prime position to influence CA3 ensemble dynamics. By mimicking presynaptic and postsynaptic activity patterns observed in vivo, we found a burst timing–dependent pattern of activity that triggered bidirectional long-term NMDAR plasticity at mossy fiber–CA3 synapses in rat hippocampal slices. This form of plasticity imparts bimodal control of mossy fiber–driven CA3 burst firing and spike temporal fidelity. Moreover, we found that mossy fiber NMDARs mediate heterosynaptic metaplasticity between mossy fiber and associational-commissural synapses. Thus, bidirectional NMDAR plasticity at mossy fiber–CA3 synapses could substantially contribute to the formation, storage and recall of CA3 cell assembly patterns.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Daw, N.W., Stein, P.S. & Fox, K. The role of NMDA receptors in information processing. Annu. Rev. Neurosci. 16, 207–222 (1993).
Hunt, D.L. & Castillo, P.E. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr. Opin. Neurobiol. 22, 496–508 (2012).
Traynelis, S.F. et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 (2010).
Schiller, J. & Schiller, Y. NMDA receptor-mediated dendritic spikes and coincident signal amplification. Curr. Opin. Neurobiol. 11, 343–348 (2001).
Herron, C.E., Lester, R.A., Coan, E.J. & Collingridge, G.L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature 322, 265–268 (1986).
Kwon, H.B. & Castillo, P.E. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 57, 108–120 (2008).
Rebola, N., Lujan, R., Cunha, R.A. & Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57, 121–134 (2008).
Nicoll, R.A. & Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876 (2005).
Jonas, P., Major, G. & Sakmann, B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J. Physiol. (Lond.) 472, 615–663 (1993).
Rebola, N., Carta, M., Lanore, F., Blanchet, C. & Mulle, C. NMDA receptor-dependent metaplasticity at hippocampal mossy fiber synapses. Nat. Neurosci. 14, 691–693 (2011).
Astori, S., Pawlak, V. & Kohr, G. Spike-timing-dependent plasticity in hippocampal CA3 neurons. J. Physiol. (Lond.) 588, 4475–4488 (2010).
Henze, D.A., Wittner, L. & Buzsaki, G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 5, 790–795 (2002).
Marr, D. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B 262, 23–81 (1971).
Jung, M.W. & McNaughton, B.L. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–182 (1993).
Caporale, N. & Dan, Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46 (2008).
Lujan, R., Nusser, Z., Roberts, J.D., Shigemoto, R. & Somogyi, P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 8, 1488–1500 (1996).
Morishita, W., Marie, H. & Malenka, R.C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat. Neurosci. 8, 1043–1050 (2005).
Peng, Y. et al. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus 20, 646–658 (2010).
Ireland, D.R. & Abraham, W.C. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses. J. Neurophysiol. 101, 1375–1385 (2009).
Abraham, W.C. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9, 387–399 (2008).
Kobayashi, K. & Poo, M.M. Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron 41, 445–454 (2004).
Kapur, A., Yeckel, M. & Johnston, D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience 107, 59–69 (2001).
Ross, W.N. Understanding calcium waves and sparks in central neurons. Nat. Rev. Neurosci. 13, 157–168 (2012).
Nakashiba, T., Buhl, D.L., McHugh, T.J. & Tonegawa, S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron 62, 781–787 (2009).
Nakazawa, K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218 (2002).
Nakazawa, K. et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38, 305–315 (2003).
Leutgeb, S., Leutgeb, J.K., Moser, M.B. & Moser, E.I. Place cells, spatial maps and the population code for memory. Curr. Opin. Neurobiol. 15, 738–746 (2005).
Torborg, C.L., Nakashiba, T., Tonegawa, S. & McBain, C.J. Control of CA3 output by feedforward inhibition despite developmental changes in the excitation-inhibition balance. J. Neurosci. 30, 15628–15637 (2010).
Mori, M., Abegg, M.H., Gahwiler, B.H. & Gerber, U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature 431, 453–456 (2004).
Kumar, A., Rotter, S. & Aertsen, A. Spiking activity propagation in neuronal networks: reconciling different perspectives on neural coding. Nat. Rev. Neurosci. 11, 615–627 (2010).
Lisman, J.E. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 20, 38–43 (1997).
Harnett, M.T., Bernier, B.E., Ahn, K.C. & Morikawa, H. Burst-timing–dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron 62, 826–838 (2009).
Sjostrom, P.J. & Nelson, S.B. Spike timing, calcium signals and synaptic plasticity. Curr. Opin. Neurobiol. 12, 305–314 (2002).
Lisman, J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl. Acad. Sci. USA 86, 9574–9578 (1989).
Valenti, O., Conn, P.J. & Marino, M.J. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J. Cell. Physiol. 191, 125–137 (2002).
Topolnik, L., Azzi, M., Morin, F., Kougioumoutzakis, A. & Lacaille, J.C. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J. Physiol. (Lond.) 575, 115–131 (2006).
Gerber, U., Gee, C.E. & Benquet, P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr. Opin. Pharmacol. 7, 56–61 (2007).
Frausto, S.F., Ito, K., Marszalec, W. & Swanson, G.T. A novel form of low-frequency hippocampal mossy fiber plasticity induced by bimodal mGlu1 receptor signaling. J. Neurosci. 31, 16897–16906 (2011).
Wang, H.X., Gerkin, R.C., Nauen, D.W. & Bi, G.Q. Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat. Neurosci. 8, 187–193 (2005).
Buzsaki, G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68, 362–385 (2010).
Bains, J.S., Longacher, J.M. & Staley, K.J. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat. Neurosci. 2, 720–726 (1999).
Lawrence, J.J. & McBain, C.J. Interneuron diversity series: containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 26, 631–640 (2003).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001).
Debanne, D., Gahwiler, B.H. & Thompson, S.M. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J. Physiol. (Lond.) 507, 237–247 (1998).
Song, S., Miller, K.D. & Abbott, L.F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 3, 919–926 (2000).
Tsukamoto, M. et al. Mossy fibre synaptic NMDA receptors trigger non-Hebbian long-term potentiation at entorhino-CA3 synapses in the rat. J. Physiol. (Lond.) 546, 665–675 (2003).
Major, G., Polsky, A., Denk, W., Schiller, J. & Tank, D.W. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol. 99, 2584–2601 (2008).
Branco, T. & Hausser, M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 69, 885–892 (2011).
Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M.M. & Kato, K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408, 584–588 (2000).
Hulme, S.R., Jones, O.D., Ireland, D.R. & Abraham, W.C. Calcium-Dependent But Action Potential-Independent BCM-Like Metaplasticity in the Hippocampus. J. Neurosci. 32, 6785–6794 (2012).
Mateos, J.M. et al. Immunocytochemical localization of the mGluR1b metabotropic glutamate receptor in the rat hypothalamus. J. Comp. Neurol. 390, 225–233 (1998).
Ferraguti, F. et al. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J. Comp. Neurol. 400, 391–407 (1998).
Uchigashima, M. et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J. Neurosci. 27, 3663–3676 (2007).
Tanaka, J. et al. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur. J. Neurosci. 12, 781–792 (2000).
Acknowledgements
We thank members of the Castillo lab (in particular, A. Chávez, T. Younts, P. Haeger and S. Makani) for their constructive discussions on the data and helpful comments on the manuscript. This work was supported by US National Institutes of Health grants to P.E.C. (R01 MH081935 and R01 DA017392). Funding for P.G.'s laboratory is provided by Ministerio de Economía y Competitividad (BFU2012-33334), Basque Country Government (IT764-13) and University of the Basque Country.
Author information
Authors and Affiliations
Contributions
D.L.H. and P.E.C. conceived the experimental design of the study. D.L.H. performed and analyzed all electrophysiological experiments. N.P. and P.G. provided electron microscopy data and analysis. D.L.H. and P.E.C. interpreted the results and wrote the paper. All authors commented on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–11 and Supplementary Tables 1 and 2 (PDF 1335 kb)
Rights and permissions
About this article
Cite this article
Hunt, D., Puente, N., Grandes, P. et al. Bidirectional NMDA receptor plasticity controls CA3 output and heterosynaptic metaplasticity. Nat Neurosci 16, 1049–1059 (2013). https://doi.org/10.1038/nn.3461
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3461
This article is cited by
-
Role of NMDAR plasticity in a computational model of synaptic memory
Scientific Reports (2021)
-
Reduced GluN1 in mouse dentate gyrus is associated with CA3 hyperactivity and psychosis-like behaviors
Molecular Psychiatry (2020)
-
A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus
Nature Neuroscience (2018)
-
Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding
Nature Reviews Neuroscience (2017)
-
Transduction of group I mGluR-mediated synaptic plasticity by β-arrestin2 signalling
Nature Communications (2016)