Abstract
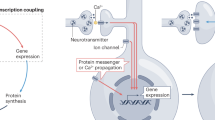
The activity-regulated cytoskeletal protein Arc (also known as Arg3.1) is required for long-term memory formation and synaptic plasticity. Arc expression is robustly induced by activity, and Arc protein localizes to both active synapses and the nucleus. Whereas its synaptic function has been examined, it is not clear why or how Arc is localized to the nucleus. We found that murine Arc nuclear expression is regulated by synaptic activity in vivo and in vitro. We identified distinct regions of Arc that control its localization, including a nuclear localization signal, a nuclear retention domain and a nuclear export signal. Arc localization to the nucleus promotes an activity-induced increase in the expression of promyelocytic leukemia nuclear bodies, which decreases GluA1 (also called Gria1) transcription and synaptic strength. We further show that Arc nuclear localization regulates homeostatic plasticity. Thus, Arc mediates the homeostatic response to increased activity by translocating to the nucleus, increasing promyelocytic leukemia protein expression and decreasing GluA1 transcription, ultimately downscaling synaptic strength.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Guzowski, J.F. et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 20, 3993–4001 (2000).
Messaoudi, E. et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J. Neurosci. 27, 10445–10455 (2007).
Park, S. et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70–83 (2008).
Waung, M.W., Pfeiffer, B.E., Nosyreva, E.D., Ronesi, J.A. & Huber, K.M. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59, 84–97 (2008).
Shepherd, J.D. et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 (2006).
Béïque, J.C., Na, Y., Kuhl, D., Worley, P.F. & Huganir, R.L. Arc-dependent synapse-specific homeostatic plasticity. Proc. Natl. Acad. Sci. USA 108, 816–821 (2011).
Plath, N. et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444 (2006).
Rao, V.R. et al. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat. Neurosci. 9, 887–895 (2006).
Pintchovski, S.A., Peebles, C.L., Kim, H.J., Verdin, E. & Finkbeiner, S. The serum response factor and a putative novel transcription factor regulate expression of the immediate-early gene Arc/Arg3.1 in neurons. J. Neurosci. 29, 1525–1537 (2009).
Ramírez-Amaya, V. et al. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J. Neurosci. 25, 1761–1768 (2005).
Kawashima, T. et al. Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc. Natl. Acad. Sci. USA 106, 316–321 (2009).
Steward, O., Wallace, C.S., Lyford, G.L. & Worley, P.F. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21, 741–751 (1998).
Huang, F., Chotiner, J.K. & Steward, O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J. Neurosci. 27, 9054–9067 (2007).
Bloomer, W.A., Vandongen, H.M. & Vandongen, A.M. Arc/Arg3.1 translation is controlled by convergent N-Methyl-D-aspartate and Gs-coupled receptor signaling pathways. J. Biol. Chem. 283, 582–592 (2008).
Panja, D. et al. Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J. Biol. Chem. 284, 31498–31511 (2009).
Greer, P.L. et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704–716 (2010).
Rodríguez, J.J. et al. Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. Eur. J. Neurosci. 21, 2384–2396 (2005).
Moga, D.E. et al. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience 125, 7–11 (2004).
Bloomer, W.A., VanDongen, H.M. & VanDongen, A.M. Activity-regulated cytoskeleton-associated protein Arc/Arg3.1 binds to spectrin and associates with nuclear promyelocytic leukemia (PML) bodies. Brain Res. 1153, 20–33 (2007).
Peebles, C.L. et al. Arc regulates spine morphology and maintains network stability in vivo. Proc. Natl. Acad. Sci. USA 107, 18173–18178 (2010).
Chowdhury, S. et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52, 445–459 (2006).
Rial Verde, E.M., Lee-Osbourne, J., Worley, P.F., Malinow, R. & Cline, H.T. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52, 461–474 (2006).
O'Brien, R.J. et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21, 1067–1078 (1998).
Borden, K.L. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol. Cell Biol. 22, 5259–5269 (2002).
Bernardi, R. & Pandolfi, P.P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 8, 1006–1016 (2007).
Regad, T., Bellodi, C., Nicotera, P. & Salomoni, P. The tumor suppressor Pml regulates cell fate in the developing neocortex. Nat. Neurosci. 12, 132–140 (2009).
Skinner, P.J. et al. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature 389, 971–974 (1997).
Chai, Y., Koppenhafer, S.L., Shoesmith, S.J., Perez, M.K. & Paulson, H.L. Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum. Mol. Genet. 8, 673–682 (1999).
Yamada, M. et al. Widespread occurrence of intranuclear atrophin-1 accumulation in the central nervous system neurons of patients with dentatorubral-pallidoluysian atrophy. Ann. Neurol. 49, 14–23 (2001).
Yamada, M. et al. Interaction between neuronal intranuclear inclusions and promyelocytic leukemia protein nuclear and coiled bodies in CAG repeat diseases. Am. J. Pathol. 159, 1785–1795 (2001).
McAllister, A.K., Katz, L.C. & Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 (1999).
Tyler, W.J., Alonso, M., Bramham, C.R. & Pozzo-Miller, L.D. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 9, 224–237 (2002).
Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 10, 86–98 (2003).
Borges, K. & Dingledine, R. Functional organization of the GluR1 glutamate receptor promoter. J. Biol. Chem. 276, 25929–25938 (2001).
St-Germain, J.R., Chen, J. & Li, Q. Involvement of PML nuclear bodies in CBP degradation through the ubiquitin-proteasome pathway. Epigenetics 3, 342–349 (2008).
Arrasate, M. & Finkbeiner, S. Automated microscope system for determining factors that predict neuronal fate. Proc. Natl. Acad. Sci. USA 102, 3840–3845 (2005).
Lee, H.R. et al. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 78, 6527–6542 (2004).
Everett, R.D. et al. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80, 7995–8005 (2006).
Gao, M. et al. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J. Neurosci. 30, 7168–7178 (2010).
Lyford, G.L. et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14, 433–445 (1995).
Zhong, S., Salomoni, P. & Pandolfi, P.P. The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2, E85–E90 (2000).
Anggono, V., Clem, R.L. & Huganir, R.L. PICK1 loss of function occludes homeostatic synaptic scaling. J. Neurosci. 31, 2188–2196 (2011).
Ibata, K., Sun, Q. & Turrigiano, G.G. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57, 819–826 (2008).
Goold, C.P. & Nicoll, R.A. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron 68, 512–528 (2010).
Sun, Q. & Turrigiano, G.G. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J. Neurosci. 31, 6800–6808 (2011).
Alberi, L. et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 69, 437–444 (2011).
Bramham, C.R. et al. The Arc of synaptic memory. Ex. Brain Res. 200, 125–140 (2010); erratum 209, 307 (2011).
Korb, E. & Finkbeiner, S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 34, 591–598 (2011).
Wang, K.H. et al. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell 126, 389–402 (2006).
Sorg, G. & Stamminger, T. Mapping of nuclear localization signals by simultaneous fusion to green fluorescent protein and to β-galactosidase. Biotechniques 26, 858–862 (1999).
Henderson, B.R. & Eleftheriou, A. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp. Cell Res. 256, 213–224 (2000).
Boutell, C., Orr, A. & Everett, R.D. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77, 8686–8694 (2003).
Brough, D., Bhatti, F. & Irvine, R.F. Mobility of proteins associated with the plasma membrane by interaction with inositol lipids. J. Cell Sci. 118, 3019–3025 (2005).
Acknowledgements
We thank R. Truant (McMaster University) for the GFP–β-gal construct, B. Henderson (University of Sydney) for the GFP-NLS-NES construct, R. Dingledine (Emory University) for the GluA1-luciferase and GluA2-luciferase constructs, P. Pandolfi (Sloan-Kettering Institute) for the eGFP-PML construct, D. Bredt (University of California San Francisco) for the eGFP-PSD95 construct, R. Everett (MRC–Universit of Glasgow Centre for Virus Research) for the PML and ICP0 construct and J. Ahn (Sungkyunkwan University School of Medicine) for the IE1 constructs. We thank members of the Finkbeiner lab, L. Jan, R. Edwards and N. Krogan for helpful suggestions and G. Howard for editorial input. E.K. was supported by a Ruth L. Kirschstein Fellowship (5 F31 MH087009). Primary support for this work was provided by the National Institute of Neurological Disease and Stroke (2 R01 NS39074), the National Institute on Aging (2 P01 AG022074) and the J. David Gladstone Institutes (S.F.), as well as the Keck Foundation (S.F.). The animal care facility was partly supported by a US National Institutes of Health Extramural Research Facilities Improvement Project (RR018928).
Author information
Authors and Affiliations
Contributions
E.K. and S.F. designed the experiments. E.K. performed experiments. C.L.W. found Pat7 and created the Arc antiserum. R.N.D. contributed to luciferase assays. K.L.L. contributed to FRAP experiments. S.F. supervised the project. E.K. and S.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1434 kb)
Rights and permissions
About this article
Cite this article
Korb, E., Wilkinson, C., Delgado, R. et al. Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nat Neurosci 16, 874–883 (2013). https://doi.org/10.1038/nn.3429
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3429
This article is cited by
-
Mechanisms and Functions of Activity-Regulated Cytoskeleton-Associated Protein in Synaptic Plasticity
Molecular Neurobiology (2023)
-
Mapping the epigenomic and transcriptomic interplay during memory formation and recall in the hippocampal engram ensemble
Nature Neuroscience (2020)
-
UPF2 leads to degradation of dendritically targeted mRNAs to regulate synaptic plasticity and cognitive function
Molecular Psychiatry (2020)
-
Amelioration of autism-like social deficits by targeting histone methyltransferases EHMT1/2 in Shank3-deficient mice
Molecular Psychiatry (2020)
-
Molecular Mechanisms of Early and Late LTP
Neurochemical Research (2019)