Abstract
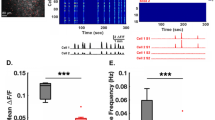
Subtle alterations in how cortical network dynamics are modulated by different behavioral states could disrupt normal brain function and underlie symptoms of neuropsychiatric disorders, including Fragile X syndrome (FXS). Using two-photon calcium imaging and electrophysiology, we recorded spontaneous neuronal ensemble activity in mouse somatosensory cortex. Unanesthetized Fmr1−/− mice exhibited abnormally high synchrony of neocortical network activity, especially during the first two postnatal weeks. Neuronal firing rates were threefold higher in Fmr1−/− mice than in wild-type mice during whole-cell recordings manifesting Up/Down states (slow-wave sleep, quiet wakefulness), probably as a result of a higher firing probability during Up states. Combined electroencephalography and calcium imaging experiments confirmed that neurons in mutant mice had abnormally high firing and synchrony during sleep. We conclude that cortical networks in FXS are hyperexcitable in a brain state–dependent manner during a critical period for experience-dependent plasticity. These state-dependent network defects could explain the intellectual, sleep and sensory integration dysfunctions associated with FXS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arieli, A., Sterkin, A., Grinvald, A. & Aertsen, A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273, 1868–1871 (1996).
Gilbert, C.D. & Sigman, M. Brain states: top-down influences in sensory processing. Neuron 54, 677–696 (2007).
Crochet, S. & Petersen, C.C. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 9, 608–610 (2006).
Ji, D. & Wilson, M.A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007).
Katz, L.C. & Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Rapin, I. & Katzman, R. Neurobiology of autism. Ann. Neurol. 43, 7–14 (1998).
Geschwind, D.H. & Levitt, P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 17, 103–111 (2007).
Portera-Cailliau, C. Which comes first in Fragile X syndrome, dendritic spine dysgenesis or defects in circuit pasticity? Neuroscientist 18, 28–44 (2012).
Olmos-Serrano, J.L. et al. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J. Neurosci. 30, 9929–9938 (2010).
Curia, G., Papouin, T., Seguela, P. & Avoli, M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex 19, 1515–1520 (2009).
Gibson, J.R., Bartley, A.F., Hays, S.A. & Huber, K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 100, 2615–2626 (2008).
Testa-Silva, G. et al. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb. Cortex 22, 1333–1342 (2012).
Miller, L.J. et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am. J. Med. Genet. 83, 268–279 (1999).
Golshani, P. et al. Internally mediated developmental desynchronization of neocortical network activity. J. Neurosci. 29, 10890–10899 (2009).
Poulet, J.F. & Petersen, C.C. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Rochefort, N.L. et al. Sparsification of neuronal activity in the visual cortex at eye opening. Proc. Natl. Acad. Sci. USA 106, 15049–15054 (2009).
Cruz-Martín, A., Crespo, M. & Portera-Cailliau, C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 30, 7793–7803 (2010).
Bureau, I., Shepherd, G.M. & Svoboda, K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knockout mice. J. Neurosci. 28, 5178–5188 (2008).
Harlow, E.G. et al. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 65, 385–398 (2010).
Stern, E.A., Maravall, M. & Svoboda, K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron 31, 305–315 (2001).
Cossart, R., Aronov, D. & Yuste, R. Attractor dynamics of network UP states in the neocortex. Nature 423, 283–288 (2003).
Steriade, M., Nunez, A. & Amzica, F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265 (1993).
Constantinople, C.M. & Bruno, R.M. Effects and mechanisms of wakefulness on local cortical networks. Neuron 69, 1061–1068 (2011).
Cowan, R.L. & Wilson, C.J. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J. Neurophysiol. 71, 17–32 (1994).
Hays, S.A., Huber, K.M. & Gibson, J.R. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J. Neurosci. 31, 14223–14234 (2011).
Li, C.Y., Poo, M.M. & Dan, Y. Burst spiking of a single cortical neuron modifies global brain state. Science 324, 643–646 (2009).
Veasey, S.C. et al. An automated system for recording and analysis of sleep in mice. Sleep 23, 1025–1040 (2000).
Tanelian, D.L., Kosek, P., Mody, I. & MacIver, M.B. The role of the GABAA receptor/chloride channel complex in anesthesia. Anesthesiology 78, 757–776 (1993).
Franks, N.P. & Lieb, W.R. Molecular and cellular mechanisms of general anaesthesia. Nature 367, 607–614 (1994).
Pfeiffer, B.E. & Huber, K.M. The state of synapses in fragile X syndrome. Neuroscientist 15, 549–567 (2009).
Bassell, G.J. & Warren, S.T. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60, 201–214 (2008).
Olshausen, B.A. & Field, D.J. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 14, 481–487 (2004).
Musumeci, S.A. et al. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia 41, 19–23 (2000).
Van der Molen, M.J. & Van der Molen, M.W. Reduced alpha and exaggerated theta power during the resting-state EEG in fragile X syndrome. Biol. Psychol. 92, 216–219 (2013).
He, C.X. & Portera-Cailliau, C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience published online, doi:10.1016/j.neuroscience.2012.03.049 (20 April 2012).
D'Hulst, C. & Kooy, R.F. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 30, 425–431 (2007).
LeBlanc, J.J. & Fagiolini, M. Autism: a “critical period” disorder? Neural Plast. 2011, 921680 (2011).
Meredith, R.M., Dawitz, J. & Kramvis, I. Sensitive time-windows for susceptibility in neurodevelopmental disorders. Trends Neurosci. 35, 335–344 (2012).
Kronk, R. et al. Prevalence, nature and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep 33, 679–687 (2010).
Zhang, J. et al. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am. J. Hum. Genet. 83, 43–52 (2008).
Bushey, D., Tononi, G. & Cirelli, C. The Drosophila fragile X mental retardation gene regulates sleep need. J. Neurosci. 29, 1948–1961 (2009).
Castro-Alamancos, M.A. Cortical up and activated states: implications for sensory information processing. Neuroscientist 15, 625–634 (2009).
Hagerman, R., Hoem, G. & Hagerman, P. Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol. Autism 1, 12 (2010).
Zhang, L., He, J., Jugloff, D.G. & Eubanks, J.H. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus 18, 294–309 (2008).
Markram, K. & Markram, H. The intense world theory: a unifying theory of the neurobiology of autism. Front. Hum. Neurosci. 4, 224 (2010).
El Idrissi, A. et al. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett. 377, 141–146 (2005).
Gentet, L.J. et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 15, 607–612 (2012).
Selby, L., Zhang, C. & Sun, Q.Q. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci. Lett. 412, 227–232 (2007).
Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33 (1994).
Golshani, P. & Portera-Cailliau, C. In vivo 2-photon calcium imaging in layer 2/3 of mice. J. Vis. Exp. 13, 681 (2008).
Pologruto, T.A., Sabatini, B.L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Guizar-Sicairos, M., Thurman, S.T. & Fienup, J.R. Efficient subpixel image registration algorithms. Opt. Lett. 33, 156–158 (2008).
Yaksi, E. & Friedrich, R.W. Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging. Nat. Methods 3, 377–383 (2006).
Mitra, P. & Bokil, H. Observed Brain Dynamics (Oxford University Press, New York, 2008).
Acknowledgements
We thank D. Buonomano, C. Houser, I. Mody and R. Mostany, as well as D. Cantu for helpful discussions and/or valuable comments on the manuscript. We also thank A. Bragin and M. Blumberg for help with EEG recordings and interpretation, J. Gornbein and D. Markovich for help with statistical analyses, and W. Greenough for providing the Fmr1−/− mice. This work was supported by grants from the National Institute of Child Health and Human Development (R01HD054453), the National Institute of Neurological Disorders and Stroke (RC1NS068093), the FRAXA Research Foundation and the Dana Foundation.
Author information
Authors and Affiliations
Contributions
J.T.G. and C.P.-C. conceived the project and designed the experiments. J.T.G., J.E.A. and P.G. conducted the experiments and analyzed the data. J.T.G. and C.P.-C. prepared the figures and wrote the manuscript. C.P.-C. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 777 kb)
Rights and permissions
About this article
Cite this article
Gonçalves, J., Anstey, J., Golshani, P. et al. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci 16, 903–909 (2013). https://doi.org/10.1038/nn.3415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3415
This article is cited by
-
Developmental delays in cortical auditory temporal processing in a mouse model of Fragile X syndrome
Journal of Neurodevelopmental Disorders (2023)
-
A working taxonomy for describing the sensory differences of autism
Molecular Autism (2023)
-
Endogenous noise of neocortical neurons correlates with atypical sensory response variability in the Fmr1−/y mouse model of autism
Nature Communications (2023)
-
Two-photon calcium imaging of neuronal activity
Nature Reviews Methods Primers (2022)
-
Brain-wide visual habituation networks in wild type and fmr1 zebrafish
Nature Communications (2022)