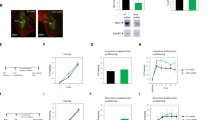
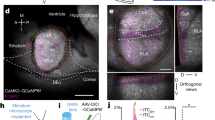
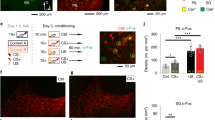
Abstract
The amygdala is essential for fear learning and expression. The central amygdala (CeA), once viewed as a passive relay between the amygdala complex and downstream fear effectors, has emerged as an active participant in fear conditioning. However, the mechanism by which CeA contributes to the learning and expression of fear is unclear. We found that fear conditioning in mice induced robust plasticity of excitatory synapses onto inhibitory neurons in the lateral subdivision of the CeA (CeL). This experience-dependent plasticity was cell specific, bidirectional and expressed presynaptically by inputs from the lateral amygdala. In particular, preventing synaptic potentiation onto somatostatin-positive neurons impaired fear memory formation. Furthermore, activation of these neurons was necessary for fear memory recall and was sufficient to drive fear responses. Our findings support a model in which fear conditioning–induced synaptic modifications in CeL favor the activation of somatostatin-positive neurons, which inhibit CeL output, thereby disinhibiting the medial subdivision of CeA and releasing fear expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Davis, M. The role of the amygdala in conditioned and unconditioned fear and anxiety. in The Amygdala (ed. Aggleton, J.P.) 213–287 (Oxford University Press, Oxford, 2000).
Davis, M. & Whalen, P.J. The amygdala: vigilance and emotion. Mol. Psychiatry 6, 13–34 (2001).
Maren, S. & Quirk, G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 5, 844–852 (2004).
LeDoux, J. The amygdala. Curr. Biol. 17, R868–874 (2007).
Ehrlich, I. et al. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 (2009).
Sigurdsson, T., Doyere, V., Cain, C.K. & LeDoux, J.E. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology 52, 215–227 (2007).
Johansen, J.P., Cain, C.K., Ostroff, L.E. & LeDoux, J.E. Molecular mechanisms of fear learning and memory. Cell 147, 509–524 (2011).
Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science 308, 83–88 (2005).
Clem, R.L. & Huganir, R.L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330, 1108–1112 (2010).
Quirk, G.J., Repa, C. & LeDoux, J.E. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039 (1995).
Rogan, M.T., Staubli, U.V. & LeDoux, J.E. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607 (1997).
McKernan, M.G. & Shinnick-Gallagher, P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390, 607–611 (1997).
Goosens, K.A. & Maren, S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci. 117, 738–750 (2003).
Paré, D., Quirk, G.J. & Ledoux, J.E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 92, 1–9 (2004).
Wilensky, A.E., Schafe, G.E., Kristensen, M.P. & LeDoux, J.E. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 26, 12387–12396 (2006).
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010).
Duvarci, S., Popa, D. & Pare, D. Central amygdala activity during fear conditioning. J. Neurosci. 31, 289–294 (2011).
Cassell, M.D., Freedman, L.J. & Shi, C. The intrinsic organization of the central extended amygdala. Ann. NY Acad. Sci. 877, 217–241 (1999).
Cassell, M.D. & Gray, T.S. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J. Comp. Neurol. 281, 320–333 (1989).
LeDoux, J.E., Iwata, J., Cicchetti, P. & Reis, D.J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988).
Dumont, E.C., Martina, M., Samson, R.D., Drolet, G. & Pare, D. Physiological properties of central amygdala neurons: species differences. Eur. J. Neurosci. 15, 545–552 (2002).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Anglada-Figueroa, D. & Quirk, G.J. Lesions of the basal amygdala block expression of conditioned fear, but not extinction. J. Neurosci. 25, 9680–9685 (2005).
Zhu, J.J., Qin, Y., Zhao, M., Van Aelst, L. & Malinow, R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455 (2002).
Zhang, F., Wang, L.P., Boyden, E.S. & Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3, 785–792 (2006).
Pitkänen, A. et al. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J. Comp. Neurol. 356, 288–310 (1995).
Ottersen, O.P. & Ben-Ari, Y. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J. Comp. Neurol. 187, 401–424 (1979).
Turner, B.H. & Herkenham, M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J. Comp. Neurol. 313, 295–325 (1991).
Zucker, R.S. & Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Fu, Y. & Shinnick-Gallagher, P. Two intra-amygdaloid pathways to the central amygdala exhibit different mechanisms of long-term potentiation. J. Neurophysiol. 93, 3012–3015 (2005).
López de Armentia, M. & Sah, P. Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala. J. Physiol. (Lond.) 581, 961–970 (2007).
Pape, H.C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Dong, S., Allen, J.A., Farrell, M. & Roth, B.L. A chemical-genetic approach for precise spatio-temporal control of cellular signaling. Mol. Biosyst. 6, 1376–1380 (2010).
Ferguson, S.M. et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci. 14, 22–24 (2011).
Huber, D., Veinante, P. & Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248 (2005).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Zhang, F., Aravanis, A.M., Adamantidis, A., de Lecea, L. & Deisseroth, K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 8, 577–581 (2007).
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Barth, A.L., Gerkin, R.C. & Dean, K.L. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 24, 6466–6475 (2004).
Chow, B.Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Quirk, G.J., Armony, J.L. & LeDoux, J.E. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19, 613–624 (1997).
He, M. et al. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 73, 35–48 (2012).
Li, B. et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470, 535–539 (2011).
Kopec, C.D. et al. A robust automated method to analyze rodent motion during fear conditioning. Neuropharmacology 52, 228–233 (2007).
López de Armentia, M. & Sah, P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J. Neurophysiol. 92, 1285–1294 (2004).
Acknowledgements
We thank R.H. Paik for expert technical assistance, K. Deisseroth and M. Mirrione for the initial help with optogenetic methods, M. Luo (National Institute of Biological Sciences, Beijing) and S.H. Shi (Memorial Sloan-Kettering Cancer Center) for the AAV-DIO-hM4Di-mCherry construct, W. Wei for help with the focal optogenetic stimulation, A. Zador (Cold Spring Harbor Laboratory) for sharing the AAV-DIO-ChR2(H134R)-YFP and AAV-DIO-Arch-GFP viruses, A. Reid (Cold Spring Harbor Laboratory) for sharing the Ai32 mice, and K. Pradhan for advice on statistic analysis. We thank S. Shea, L. Van Aelst and R. Malinow for critical reading of the manuscript, and members of the Li laboratory for discussions. This study was supported by the US National Institutes of Health (5R01MH091903-03 to B.L. and 5U01MH078844-05 to Z.J.H.), the Dana Foundation (B.L.) and the National Alliance for Research on Schizophrenia and Depression (B.L. and Z.J.H.).
Author information
Authors and Affiliations
Contributions
H.L. and M.A.P. performed the experiments. H.L., M.A.P. and B.L. analyzed the data. H.T. and Z.J.H. provided critical reagents and advice. C.D.K. developed the MatLab programs for statistical (bootstrap) and behavioral analysis. H.L., M.A.P. and B.L. designed the study. B.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 37348 kb)
Supplementary Movie 1
ChR2 activation of SOM+ neurons in CeL induces freezing responses in a naive mouse. Movie (1x) shows the behavior of one of the "ChR2" mice described in Figure 7c. The precise timing of the two 20-s blue light pulses delivered bilaterally into CeL (for the activation of ChR2) is indicated in the lower right corner (Light ON, twice). Freezing responses were reversibly induced upon the delivery of light pulses. (MOV 24103 kb)
Supplementary Movie 2
Arch inhibition of SOM+ neurons in CeL attenuates conditioned freezing responses in a fear-conditioned mouse. Movie (1×) shows the behavior of one of the Arch mice described in Figure 7e. A two-trial fear memory testing session 24 h after fear conditioning is shown. In the first trial a 20-s tone (CS) was presented during the delivery of a green light bilaterally into CeL (for the activation of Arch). The green light can be seen as a bright spot on the mouse's head. In the second trial the CS was presented in the absence of the green light. The timing of each trial is indicated in the lower right corner. A house light was kept on throughout the testing sessions to mask the green laser light. (MOV 41833 kb)
Rights and permissions
About this article
Cite this article
Li, H., Penzo, M., Taniguchi, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16, 332–339 (2013). https://doi.org/10.1038/nn.3322
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3322
This article is cited by
-
Top-down control of flight by a non-canonical cortico-amygdala pathway
Nature (2024)
-
Plastic and stimulus-specific coding of salient events in the central amygdala
Nature (2023)
-
Topographic representation of current and future threats in the mouse nociceptive amygdala
Nature Communications (2023)
-
Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala
Molecular Psychiatry (2023)
-
Selective Disruption of Perineuronal Nets in Mice Lacking Crtl1 is Sufficient to Make Fear Memories Susceptible to Erasure
Molecular Neurobiology (2023)