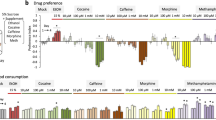
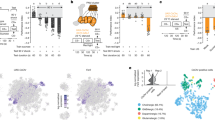
Abstract
The rewarding properties of drugs contribute to the development of abuse and addiction. We developed a new assay for investigating the motivational properties of ethanol in the genetically tractable model Drosophila melanogaster. Flies learned to associate cues with ethanol intoxication and, although transiently aversive, the experience led to a long-lasting attraction for the ethanol-paired cue, implying that intoxication is rewarding. Temporally blocking transmission in dopaminergic neurons revealed that flies require activation of these neurons to express, but not develop, conditioned preference for ethanol-associated cues. Moreover, flies acquired, consolidated and retrieved these rewarding memories using distinct sets of neurons in the mushroom body. Finally, mutations in scabrous, encoding a fibrinogen-related peptide that regulates Notch signaling, disrupted the formation of memories for ethanol reward. Our results thus establish that Drosophila can be useful for understanding the molecular, genetic and neural mechanisms underling the rewarding properties of ethanol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hyman, S.E., Malenka, R.C. & Nestler, E.J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565–598 (2006).
Tzschentke, T.M. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 12, 227–462 (2007).
Rodan, A.R. & Rothenfluh, A. The genetics of behavioral alcohol responses in Drosophila. Int. Rev. Neurobiol. 91, 25–51 (2010).
Baker, N.E., Mlodzik, M. & Rubin, G.M. Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science 250, 1370–1377 (1990).
Powell, P.A., Wesley, C., Spencer, S. & Cagan, R.L. Scabrous complexes with Notch to mediate boundary formation. Nature 409, 626–630 (2001).
Nehring, L.C., Miyamoto, A., Hein, P.W., Weinmaster, G. & Shipley, J.M. The extracellular matrix protein MAGP-2 interacts with Jagged1 and induces its shedding from the cell surface. J. Biol. Chem. 280, 20349–20355 (2005).
Miyamoto, A., Lau, R., Hein, P.W., Shipley, J.M. & Wienmaster, G. Microfibrillar proteins MAGP-1 and MAGP-2 induce Notch1 extracellular domain dissociation and receptor activation. J. Biol. Chem. 281, 10089–10097 (2006).
Louvi, A. & Artavanis-Tsakonas, S. Notch signaling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 (2006).
Wang, Y. et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. USA 101, 9458–9462 (2004).
Costa, R.M., Honjo, T. & Silva, A.J. Learning and memory deficits in Notch mutant mice. Curr. Biol. 13, 1348–1354 (2003).
Presente, A., Boyles, R.S., Serway, C.N., de Belle, S. & Andres, A. Notch is required for long-term memory in Drosophila. Proc. Natl. Acad. Sci. USA 101, 1764–1768 (2004).
Ge, X. et al. Notch signaling in Drosophila long-term memory formation. Proc. Natl. Acad. Sci. USA 101, 10172–10176 (2004).
Phillips, T.J. & Shen, E.H. Neurochemical basis of locomotion and ethanol stimulant effects. Int. Rev. Neurobiol. 39, 243–282 (1996).
Deadwyler, S.A. Electrophysiological correlates of abused drugs: relation to natural rewards. Ann. NY Acad. Sci. 1187, 140–147 (2010).
Bainton, R.J. et al. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 10, 187–194 (2000).
Kong, E.C. et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 5, e9954 (2010).
Kitamoto, T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J. Neurogenet. 16, 205–228 (2002).
Friggi-Grelin, F. et al. Targeted expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627 (2003).
Li, H., Chaney, S., Forte, M. & Hirsh, J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 10, 211–214 (2000).
Dhonnchadha, B.A.N. & Cunningham, K.A. Serotonergic mechanisms in addiction-related memories. Behav. Brain Res. 195, 29–52 (2008).
Krashes, M.J., Keene, A.C., Leung, B., Armstrong, J.D. & Waddell, S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53, 103–115 (2007).
Krashes, M.J. et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427 (2009).
Claridge-Chang, A. et al. Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415 (2009).
LaFerriere, H. et al. Genetic dissociation of ethanol sensitivity and memory formation in Drosophila melanogaster. Genetics 178, 1895–1902 (2008).
Hu, X., Lee, E.C. & Baker, N.E. Molecular analysis of scabrous mutant alleles from Drosophila melanogaster indicates a secreted protein with two functional domains. Genetics 141, 607–617 (1995).
Devineni, A.V. & Heberlein, U. Preferential ethanol consumption in Drosophila models features of addiction. Curr. Biol. 19, 2126–2132 (2009).
Laviolette, S.R. & van der Kooy, D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat. Rev. Neurosci. 5, 55–65 (2004).
Pautassi, R.M., Molina, J.C. & Spear, N. Infant rats exhibit aversive learning mediated by ethanol's orosensory effects but are positively reinforced by ethanol's post-ingestive effects. Pharmacol. Biochem. Behav. 88, 393–402 (2008).
Cunningham, C.L. et al. Ethanol-conditioned place preference reduced in dopamine D2 receptor–deficient mice. Pharmacol. Biochem. Behav. 67, 693–699 (2000).
Risinger, F.O., Freeman, P.A., Greengard, P. & Fienberg, A.A. Motivational effects of ethanol in DARPP-32 knock-out mice. J. Neurosci. 21, 340–348 (2001).
Walker, B.M. & Ettenberg, A. Intracerebroventricular ethanol-induced conditioned place preference are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav. Neurosci. 121, 401–410 (2007).
Gremel, C.M. & Cunningham, C.L. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology 34, 1443–1453 (2008).
Schultz, W. Getting formal with dopamine and reward. Neuron 36, 241–263 (2002).
Schwaerzel, M. et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502 (2003).
Kim, Y.C., Lee, H.G. & Han, K.A. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 27, 7640–7647 (2007).
Zars, T., Fischer, M., Schultz, R. & Heisenberg, M. Localization of a short-term memory in Drosophila. Science 288, 672–675 (2000).
Dubnau, J., Grady, L., Kitamoto, T. & Tully, T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 411, 476–480 (2001).
McGuire, S.E., Le, P.T. & Davis, R.L. The role of Drosophila mushroom body signaling in olfactory memory. Science 293, 1330–1333 (2001).
King, I. et al. Drosophila tao controls mushroom body development and ethanol-stimulated behavior through par-1. J. Neurosci. 31, 1139–1148 (2011).
Akalal, D.B., Yu, D. & Davis, R.L. A late-phase, long-term memory trace forms in the γ neurons of Drosophila mushroom bodies after olfactory classical conditioning. J. Neurosci. 30, 16699–166708 (2010).
Isabel, G., Pascual, A. & Preat, T. Exclusive consolidated memory phases in Drosophila. Science 304, 1024–1027 (2004).
Brand, A.H., Manuokian, A.S. & Perrimon, N. Ectopic expression in Drosophila. Methods Cell Biol. 44, 635–654 (1994).
Berger, K.H. et al. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol. Clin. Exp. Res. 32, 895–908 (2008).
Hardie, S.L. & Hirsh, J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extract by high performance liquid chromatography. J. Neurosci. Methods 153, 243–249 (2006).
Aso, Y. et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 23, 156–172 (2009).
Acknowledgements
We thank S. Waddell, J. Levine, M. Sokolowski, A. Barron and members of the Heberlein laboratory for reagents, input and advice, and S. Birman (Developmental Biology Institute of Marseilles-Luminy), F. Wolf (Gallo Institute), S. Sweeney (University of York), K. Kaiser (Glasgow University), N. Baker (Albert Einstein College of Medicine) and the Bloomington Stock Center for flies. Funding was provided by a Heart and Stroke Foundation of Canada Research Fellowship to K.R.K. and the US National Institutes of Health to U.H.
Author information
Authors and Affiliations
Contributions
K.R.K. conceived, conducted and interpreted the experiments, performed data analysis, and co-wrote the paper. R.A. assisted with the behavior experiments. Z.M. conducted control experiments. J.H. performed high-performance liquid chromatography experiments. U.H. conceived and interpreted experiments and co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–5 (PDF 2924 kb)
Rights and permissions
About this article
Cite this article
Kaun, K., Azanchi, R., Maung, Z. et al. A Drosophila model for alcohol reward. Nat Neurosci 14, 612–619 (2011). https://doi.org/10.1038/nn.2805
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2805
This article is cited by
-
Dopamine determines how reward overrides risk
Nature (2023)
-
Dopaminergic systems create reward seeking despite adverse consequences
Nature (2023)
-
PT150 blocks the rewarding properties of ethanol and attenuates ethanol-induced reduction of egg laying in Coturnix quail
Psychopharmacology (2023)
-
The Role of the Dopamine Transporter in the Effects of Amphetamine on Sleep and Sleep Architecture in Drosophila
Neurochemical Research (2022)
-
The role of mGlu4 receptors within the nucleus accumbens in acquisition and expression of morphine-induced conditioned place preference in male rats
BMC Neuroscience (2021)