Abstract
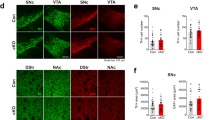
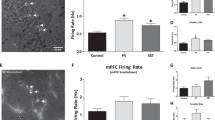
Mesencephalic-diencephalic dopaminergic neurons control locomotor activity and emotion and are affected in neurodegenerative and psychiatric diseases. The homeoprotein Otx2 is restricted to ventral tegmental area (VTA) neurons that are prevalently complementary to those expressing Girk2 and glycosylated active form of the dopamine transporter (Dat). High levels of glycosylated Dat mark neurons with efficient dopamine uptake and pronounced vulnerability to Parkinsonian degeneration. We found that Otx2 controls neuron subtype identity by antagonizing molecular and functional features of dorsal-lateral VTA, such as Girk2 and Dat expression. Otx2 limited the number of VTA neurons with efficient dopamine uptake and conferred resistance to the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-HCl (MPTP) neurotoxin. Ectopic Otx2 expression also provided neurons of the substantia nigra with efficient neuroprotection to MPTP. These findings indicate that Otx2 is required to specify neuron subtype identity in VTA and may antagonize vulnerability to the Parkinsonian toxin MPTP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Björklund, A. & Lindvall, O. Dopamine-containing systems in the CNS. in Handbook of Chemical Neuroanatomy: Classical Transmitters in the CNS (eds. Biörklund, A. and Hökfelt, T.) 55–121 (Elsevier, Amsterdam, 1984).
Björklund, L.M. & Isacson, O. Regulation of dopamine cell type and transmitter function in fetal and stem cell transplantation for Parkinson's disease. Prog. Brain Res. 138, 411–420 (2002).
Dahlström, A. & Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 62, 1–55 (1964).
Smidt, M.P. & Burbach, J.P. How to make a mesodiencephalic dopaminergic neuron. Nat. Rev. Neurosci. 8, 21–32 (2007).
Marín, F., Herrero, M.T., Vyas, S. & Puelles, L. Ontogeny of tyrosine hydroxylase mRNA expression in mid- and forebrain: neuromeric pattern and novel positive regions. Dev. Dyn. 234, 709–717 (2005).
Omodei, D. et al. Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development 135, 3459–3470 (2008).
Thompson, L., Barraud, P., Andersson, E., Kirik, D. & Björklund, A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression and efferent projections. J. Neurosci. 25, 6467–6477 (2005).
Jellinger, K.A. The pathology of Parkinson′s disease. Adv. Neurol. 86, 55–72 (2001).
Prakash, N. & Wurst, W. Development of dopaminergic neurons in the mammalian brain. Cell. Mol. Life Sci. 63, 187–206 (2006).
Simeone, A. Genetic control of dopaminergic neuron differentiation. Trends Neurosci. 28, 62–65 (2005).
Saucedo-Cardenas, O. et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA 95, 4013–4018 (1998).
Jankovic, J., Chen, S. & Le, W.D. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog. Neurobiol. 77, 128–138 (2005).
Smidt, M.P. et al. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat. Neurosci. 3, 337–341 (2000).
Smidt, M.P. et al. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development 131, 1145–1155 (2004).
Kele, J. et al. Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 133, 495–505 (2006).
Ferri, A.L. et al. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134, 2761–2769 (2007).
Andersson, E., Jensen, J.B., Parmar, M., Guillemot, F. & Björklund, A. Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development 133, 507–516 (2006).
Andersson, E. et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124, 393–405 (2006).
Ono, Y. et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134, 3213–3225 (2007).
van den Munckhof, P. et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130, 2535–2542 (2003).
Nunes, I., Tovmasian, L.T., Silva, R.M., Burke, R.E. & Goff, S.P. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc. Natl. Acad. Sci. USA 100, 4245–4250 (2003).
Simon, H.H., Saueressig, H., Wurst, W., Goulding, M.D. & O'Leary, D.D. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 21, 3126–3134 (2001).
Sonnier, L. et al. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J. Neurosci. 27, 1063–1071 (2007).
Sgadò, P. et al. Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice. Proc. Natl. Acad. Sci. USA 103, 15242–15247 (2006).
Zetterström, R.H. et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science 276, 248–250 (1997).
Castelo-Branco, G. et al. Differential regulation of dopaminergic neuron development by Wnt-1, Wnt-3a and Wnt-5a. Proc. Natl. Acad. Sci. USA 100, 12747–12752 (2003).
Puelles, E. et al. Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat. Neurosci. 6, 453–460 (2003).
Puelles, E. et al. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 131, 2037–2048 (2004).
Prakash, N. et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development 133, 89–98 (2006).
Afonso-Oramas, D. et al. Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson's disease. Neurobiol. Dis. 36, 494–508 (2009).
Dauer, W. & Przedborski, S. Parkinson's disease: mechanisms and models. Neuron 39, 889–909 (2003).
Jackson-Lewis, V., Jakowec, M., Burke, R.E. & Przedborski, S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4, 257–269 (1995).
Blum, D. et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog. Neurobiol. 65, 135–172 (2001).
Chung, C.Y. et al. Cell type–specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum. Mol. Genet. 14, 1709–1725 (2005).
Greene, J.G., Dingledine, R. & Greenamyre, J.T. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol. Dis. 18, 19–31 (2005).
Murer, G. et al. An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience 80, 345–357 (1997).
Schein, J.C., Hunter, D.D. & Roffler-Tarlov, S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev. Biol. 204, 432–450 (1998).
Di Salvio, M., Di Giovannantonio, L.G., Omodei, D., Acampora, D. & Simeone, A. Otx2 expression is restricted to dopaminergic neurons of the ventral tegmental area in the adult brain. Int. J. Dev. Biol. 54, 939–945 (2010).
Liang, C.L., Sinton, C.M., Sonsalla, P.K. & German, D.C. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration 5, 313–318 (1996).
Roffler-Tarlov, S., Martin, B., Graybiel, A.M. & Kauer, J.S. Cell death in the midbrain of the murine mutation weaver. J. Neurosci. 16, 1819–1826 (1996).
Bäckman, C.M. et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44, 383–390 (2006).
Acampora, D., Di Giovannantonio, L.G., Di Salvio, M., Mancuso, P. & Simeone, A. Selective inactivation of Otx2 mRNA isoforms reveals isoform-specific requirement for visceral endoderm anteriorization and head morphogenesis and highlights cell diversity in the visceral endoderm. Mech. Dev. 126, 882–897 (2009).
Kittappa, R., Chang, W.W., Awatramani, R.B. & McKay, R.D. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 5, e325 (2007).
Li, L.B. et al. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 279, 21012–21020 (2004).
Torres, G.E. et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 278, 2731–2739 (2003).
Bezard, E. et al. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp. Neurol. 155, 268–273 (1999).
Pifl, C., Giros, B. & Caron, M.G. Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J. Neurosci. 13, 4246–4253 (1993).
Agoston, Z. & Schulte, D. Meis2 competes with the Groucho co-repressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrain-hindbrain boundary organizer. Development 136, 3311–3322 (2009).
Kadkhodaei, B. et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J. Neurosci. 29, 15923–15932 (2009).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Acknowledgements
The authors would like to thank D. Grieco and T. Russo for helpful discussions and criticisms on the manuscript, S. Casola for the R26 targeting vector, G. Corte for the Otx2 antibody and the staff of the CEINGE animal house for excellent animal care. We are grateful to E. Bricola for typing the manuscript. This work was supported by the FP7 for the project mdDA NEURODEV (222999) to A.S. and W.W., the FP6 project for the EUTRACC Integrated Project (LSHG-CT-2007-037445) to A.S. and W.W., the 'Stem Cell Project' of Fondazione Roma and the Italian Association for Cancer Research to A.S., and the Federal Ministry of Education and Research in the framework of the National Genome Research Network (NGFN+ Functional Genomics of Parkinson Disease FKZ 01GS08174) to W.W.
Author information
Authors and Affiliations
Contributions
M.D.S. and L.G.D.G. performed the experiments, D.A. generated the Otx2 mutant mice, R.P. performed the statistical analysis, D.O. and N.P. contributed to the phenotypic analysis, W.W. contributed to the interpretation of results and the writing of the manuscript, and A.S. designed and interpreted the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–3 (PDF 1100 kb)
Rights and permissions
About this article
Cite this article
Di Salvio, M., Di Giovannantonio, L., Acampora, D. et al. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci 13, 1481–1488 (2010). https://doi.org/10.1038/nn.2661
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2661
This article is cited by
-
Development, wiring and function of dopamine neuron subtypes
Nature Reviews Neuroscience (2023)
-
A core-satellite-like nanoassembly reverses a decisive tyrosine hydroxylase loss in degenerative dopaminergic neurons
Nano Research (2023)
-
Maintenance of mitochondrial integrity in midbrain dopaminergic neurons governed by a conserved developmental transcription factor
Nature Communications (2022)
-
Non-cell-autonomous OTX2 transcription factor regulates anxiety-related behavior in the mouse
Molecular Psychiatry (2021)
-
Nolz1 expression is required in dopaminergic axon guidance and striatal innervation
Nature Communications (2020)