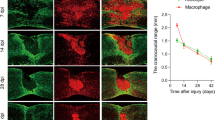
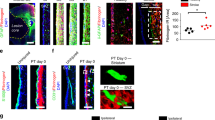
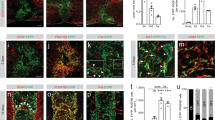
Abstract
Central nervous system (CNS) injury transforms naive astrocytes into reactive astrocytes, which eventually become scar-forming astrocytes that can impair axonal regeneration and functional recovery. This sequential phenotypic change, known as reactive astrogliosis, has long been considered unidirectional and irreversible. However, we report here that reactive astrocytes isolated from injured spinal cord reverted in retrograde to naive astrocytes when transplanted into a naive spinal cord, whereas they formed astrocytic scars when transplanted into injured spinal cord, indicating the environment-dependent plasticity of reactive astrogliosis. We also found that type I collagen was highly expressed in the spinal cord during the scar-forming phase and induced astrocytic scar formation via the integrin–N-cadherin pathway. In a mouse model of spinal cord injury, pharmacological blockade of reactive astrocyte–type I collagen interaction prevented astrocytic scar formation, thereby leading to improved axonal regrowth and better functional outcomes. Our findings reveal environmental cues regulating astrocytic fate decisions, thereby providing a potential therapeutic target for CNS injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McDonald, J.W. & Sadowsky, C. Spinal-cord injury. Lancet 359, 417–425 (2002).
Okada, S. et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834 (2006).
Silver, J. & Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004).
Karimi-Abdolrezaee, S. & Billakanti, R. Reactive astrogliosis after spinal cord injury—beneficial and detrimental effects. Mol. Neurobiol. 46, 251–264 (2012).
Ridet, J.L., Malhotra, S.K., Privat, A. & Gage, F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 20, 570–577 (1997).
Buss, A. et al. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127, 34–44 (2004).
Anderson, M.A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Liddelow, S.A. & Barres, B.A. Regeneration: not everything is scary about a glial scar. Nature 532, 182–183 (2016).
Windle, W.F., Clemente, C.D. & Chambers, W.W. Inhibition of formation of a glial barrier as a means of permitting a peripheral nerve to grow into the brain. J. Comp. Neurol. 96, 359–369 (1952).
Freeman, L.W. Return of spinal cord function in mammals after transecting lesions. Ann. NY Acad. Sci. 58, 564–569 (1954).
Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Cregg, J.M. et al. Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197–207 (2014).
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007).
Renault-Mihara, F. et al. Beneficial compaction of spinal cord lesion by migrating astrocytes through glycogen synthase kinase-3 inhibition. EMBO Mol. Med. 3, 682–696 (2011).
Vázquez-Chona, F. & Geisert, E.E.J. Jr. N-cadherin at the glial scar in the rat. Brain Res. 838, 45–50 (1999).
McKillop, W.M., Dragan, M., Schedl, A. & Brown, A. Conditional Sox9 ablation reduces chondroitin sulfate proteoglycan levels and improves motor function following spinal cord injury. Glia 61, 164–177 (2013).
Mann, B. et al. Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 96, 1603–1608 (1999).
Blasi, F. & Carmeliet, P. uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 3, 932–943 (2002).
Takeuchi, K. et al. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat. Commun. 4, 2740 (2013).
Hagino, S. et al. Slit and glypican-1 mRNAs are coexpressed in the reactive astrocytes of the injured adult brain. Glia 42, 130–138 (2003).
Ilieva, H., Polymenidou, M. & Cleveland, D.W. Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 187, 761–772 (2009).
von Karstedt, S. et al. Cancer cell–autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell 27, 561–573 (2015).
Kumamaru, H. et al. Direct isolation and RNA–seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat. Commun. 3, 1140 (2012).
Kumamaru, H. et al. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells 31, 1535–1547 (2013).
Takeichi, M. The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 8, 11–20 (2007).
Tran, M.D., Wanner, I.B. & Neary, J.T. Purinergic receptor signaling regulates N-cadherin expression in primary astrocyte cultures. J. Neurochem. 105, 272–286 (2008).
Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Previtali, S.C., Archelos, J.J. & Hartung, H.P. Modulation of the expression of integrins on glial cells during experimental autoimmune encephalomyelitis. A central role for TNF-α. Am. J. Pathol. 151, 1425–1435 (1997).
Yonezawa, T. et al. Type IV collagen induces expression of thrombospondin-1 that is mediated by integrin α1β1 in astrocytes. Glia 58, 755–767 (2010).
Doyle, J.P. et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135, 749–762 (2008).
Garg, A. et al. Non-enzymatic dissociation of human mesenchymal stromal cells improves chemokine-dependent migration and maintains immunosuppressive function. Cytotherapy 16, 545–559 (2014).
Yokota, K. et al. Engrafted neural stem/progenitor cells promote functional recovery through synapse reorganization with spared host neurons after spinal cord injury. Stem Cell Rep. 5, 264–277 (2015).
Faulkner, J.R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004).
Rolls, A. et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 5, e171 (2008).
Rolls, A., Shechter, R. & Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10, 235–241 (2009).
Shechter, R., Raposo, C., London, A., Sagi, I. & Schwartz, M. The glial scar–monocyte interplay: a pivotal resolution phase in spinal cord repair. PLoS One 6, e27969 (2011).
Brionne, T.C., Tesseur, I., Masliah, E. & Wyss-Coray, T. Loss of TGF-β1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145 (2003).
Abe, K., Chu, P.J., Ishihara, A. & Saito, H. Transforming growth factor-β1 promotes re-elongation of injured axons of cultured rat hippocampal neurons. Brain Res. 723, 206–209 (1996).
Lutton, C. et al. Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. J. Neurotrauma 29, 957–970 (2012).
DePaul, M.A., Lin, C.Y., Silver, J. & Lee, Y.S. Peripheral nerve transplantation combined with acidic fibroblast growth factor and chondroitinase induces regeneration and improves urinary function in complete spinal cord transected adult mice. PLoS One 10, e0139335 (2015).
Göritz, C. et al. A pericyte origin of spinal cord scar tissue. Science 333, 238–242 (2011).
Shintani, Y., Wheelock, M.J. & Johnson, K.R. Phosphoinositide-3 kinase–Rac1–c-Jun NH2-terminal kinase signaling mediates collagen I–induced cell scattering and up-regulation of N-cadherin expression in mouse mammary epithelial cells. Mol. Biol. Cell 17, 2963–2975 (2006).
Shintani, Y., Hollingsworth, M.A., Wheelock, M.J. & Johnson, K.R. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH2-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 66, 11745–11753 (2006).
Le Dréau, G. et al. NOV/CCN3 upregulates CCL2 and CXCL1 expression in astrocytes through β1 and β5 integrins. Glia 58, 1510–1521 (2010).
Cao, J., Wang, J.S., Ren, X.H. & Zang, W.D. Spinal sample showing p-JNK and p38 associated with the pain signaling transduction of glial cell in neuropathic pain. Spinal Cord 53, 92–97 (2015).
Gao, K. et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia 61, 2063–2077 (2013).
Repici, M. et al. Specific inhibition of the JNK pathway promotes locomotor recovery and neuroprotection after mouse spinal cord injury. Neurobiol. Dis. 46, 710–721 (2012).
Kanemaru, K. et al. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc. Natl. Acad. Sci. USA 110, 11612–11617 (2013).
Péglion, F. & Etienne-Manneville, S. N-cadherin expression level as a critical indicator of invasion in non-epithelial tumors. Cell Adh Migr. 6, 327–332 (2012).
Bush, T.G. et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23, 297–308 (1999).
Silver, J. The glial scar is more than just astrocytes. Exp. Neurol. 286, 147–149 (2016).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997).
Sanai, N. et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427, 740–744 (2004).
Takahashi, S. et al. C-type lectin–like domain and fibronectin-like type II domain of phospholipase A2 receptor 1 modulate binding and migratory responses to collagen. FEBS Lett. 589, 829–835 (2015).
Hayashi, M. et al. Chd5 regulates MuERV-L/MERVL expression in mouse embryonic stem cells via H3K27me3 modification and histone H3.1/H3.2. J. Cell. Biochem. 117, 780–792 (2016).
Harada, A. et al. Incorporation of histone H3.1 suppresses the lineage potential of skeletal muscle. Nucleic Acids Res. 43, 775–786 (2015).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA–seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Dennis, G. Jr. et al. DAVID: Database for Annotation, Visualization, and integrated Discovery. Genome Biol. 4, P3 (2003).
Hashimshony, T. et al. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 17, 77 (2016).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA–seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank T. Tachibana (Osaka City University) for the gift of anti-nestin antibody. This study was supported by a Grant-in-Aid for Young Scientist (A) (25713053) (S.O.); Challenging Exploratory Research from the Ministry of Education, Science, and Sports (16K15668) (S.O.); a Grant-in-Aid for Young Scientist (B) (16K20059) (K. Kobayakawa); Scientific Research on Innovative Areas (Comprehensive Brain Science Network and Foundation of Synapse Neurocircuit Pathology) (K. Kobayakawa); and research foundations from the general insurance association of Japan (M.H.).
Author information
Authors and Affiliations
Contributions
M.H. designed and performed most of the experiments with technical help from H.K., T.S., and S.Y. Y.O. performed the RNA–seq analysis. K.Y. and K. Kijima analyzed the data from LMD. K. Kobayakawa, K.H., and Y.N. designed the studies and supervised the overall project. S.O. designed the studies, supervised the overall project, and performed the final manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Table
Supplementary Figures 1–13 and Supplementary Table 1 (PDF 18120 kb)
Rights and permissions
About this article
Cite this article
Hara, M., Kobayakawa, K., Ohkawa, Y. et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin–N-cadherin pathway after spinal cord injury. Nat Med 23, 818–828 (2017). https://doi.org/10.1038/nm.4354
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4354
This article is cited by
-
Exosome-mediated repair of spinal cord injury: a promising therapeutic strategy
Stem Cell Research & Therapy (2024)
-
Corticospinal tract: a new hope for the treatment of post-stroke spasticity
Acta Neurologica Belgica (2024)
-
M2 Microglia-derived Exosomes Promote Spinal Cord Injury Recovery in Mice by Alleviating A1 Astrocyte Activation
Molecular Neurobiology (2024)
-
LPAR6 Participates in Neuropathic Pain by Mediating Astrocyte Cells via ROCK2/NF-κB Signal Pathway
Molecular Neurobiology (2024)
-
Atractylenolide I Suppresses A1 Astrocyte Activation to Improve Depression in Mice
Molecular Neurobiology (2024)