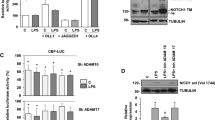
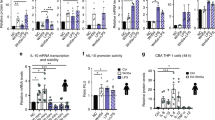
Abstract
The stimulation of Toll-like receptors (TLRs) on macrophages triggers production of the cytokine tumor necrosis factor (TNF). TNF production occurs within 1 h of TLR stimulation and is sustained for 1 d. Here we document a function for the TNF family member 4-1BB ligand (4-1BBL) in sustaining TLR-induced TNF production. TLR signaling induced 4-1BBL, and 4-1BBL interacted with TLRs on the macrophage surface. The influence of 4-1BBL on TNF production was independent of its receptor (4-1BB) and did not require the adaptors MyD88 or TRIF. It did not influence TLR4-induced activation of transcription factor NF-κB (an early response) but was required for TLR4-induced activation of transcription factors CREB and C/EBP (a late event). Transient TLR4-MyD88 complexes appeared during the first hour after lipopolysaccharide stimulation, and TLR4–4-1BBL interactions were detected between 2 h and 8 h after lipopolysaccharide stimulation. Our results indicate that two different TLR4 complexes sequentially form and selectively control early and late TNF production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cohen, J. The immunopathogenesis of sepsis. Nature 420, 885–891 (2002).
Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 (2004).
Janeway, C.A., Jr. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Goodwin, R.G. et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 23, 2631–2641 (1993).
Alderson, M.R. et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur. J. Immunol. 24, 2219–2227 (1994).
Watts, T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23, 23–68 (2005).
Xu, Y. et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000).
Futagawa, T. et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 14, 275–286 (2002).
Laderach, D., Wesa, A. & Galy, A. 4-1BB-ligand is regulated on human dendritic cells and induces the production of IL-12. Cell. Immunol. 226, 37–44 (2003).
Langstein, J. et al. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J. Immunol. 160, 2488–2494 (1998).
Shin, H.H., Lee, E.A., Kim, S.J., Kwon, B.S. & Choi, H.S. A signal through 4-1BB ligand inhibits receptor for activation of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis by increasing interferon (IFN)-β production. FEBS Lett. 580, 1601–1606 (2006).
Vassalli, P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 10, 411–452 (1992).
Ameloot, P., Declercq, W., Fiers, W., Vandenabeele, P. & Brouckaert, P. Heterotrimers formed by tumor necrosis factors of different species or muteins. J. Biol. Chem. 276, 27098–27103 (2001).
Locksley, R.M., Killeen, N. & Lenardo, M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Zhang, H., Tay, P.N., Cao, W., Li, W. & Lu, J. Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling. FEBS Lett. 532, 171–176 (2002).
Lee, H.K., Dunzendorfer, S. & Tobias, P.S. Cytoplasmic domain-mediated dimerizations of toll-like receptor 4 observed by β-lactamase enzyme fragment complementation. J. Biol. Chem. 279, 10564–10574 (2004).
Vinay, D.S. et al. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J. Immunol. 173, 4218–4229 (2004).
Seo, S.K. et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med. 10, 1088–1094 (2004).
Beutler, B. et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 316, 552–554 (1985).
Han, J., Beutler, B. & Huez, G. Complex regulation of tumor necrosis factor mRNA turnover in lipopolysaccharide-activated macrophages. Biochim. Biophys. Acta 1090, 22–28 (1991).
Akira, S. et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 9, 1897–1906 (1990).
Pope, R., Mungre, S., Liu, H. & Thimmapaya, B. Regulation of TNF-α expression in normal macrophages: the role of C/EBPβ. Cytokine 12, 1171–1181 (2000).
Tsai, E.Y. et al. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor α promoter in vivo. Mol. Cell. Biol. 20, 6084–6094 (2000).
Roach, S.K., Lee, S.B. & Schorey, J.S. Differential activation of the transcription factor cyclic AMP response element binding protein (CREB) in macrophages following infection with pathogenic and nonpathogenic mycobacteria and role for CREB in tumor necrosis factor α production. Infect. Immun. 73, 514–522 (2005).
Klaffenbach, D. et al. Regulation and signal transduction of toll-like receptors in human chorioncarcinoma cell lines. Am. J. Reprod. Immunol. 53, 77–84 (2005).
Yeo, S.J., Yoon, J.G., Hong, S.C. & Yi, A.K. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. J. Immunol. 170, 1052–1061 (2003).
Hornung, V. et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537 (2002).
Husebye, H. et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 25, 683–692 (2006).
Nomura, F. et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164, 3476–3479 (2000).
Matsuguchi, T., Musikacharoen, T., Ogawa, T. & Yoshikai, Y. Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J. Immunol. 165, 5767–5772 (2000).
An, H. et al. Involvement of ERK, p38 and NF-κB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology 106, 38–45 (2002).
Nhu, Q.M., Cuesta, N. & Vogel, S.N. Transcriptional regulation of lipopolysaccharide (LPS)-induced Toll-like receptor (TLR) expression in murine macrophages: role of interferon regulatory factors 1 (IRF-1) and 2 (IRF-2). J. Endotoxin Res. 12, 285–295 (2006).
Pollok, K.E. et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 150, 771–781 (1993).
Melero, I. et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 3, 682–685 (1997).
Halstead, E.S., Mueller, Y.M., Altman, J.D. & Katsikis, P.D. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat. Immunol. 3, 536–541 (2002).
Bertram, E.M., Lau, P. & Watts, T.H. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168, 3777–3785 (2002).
DeBenedette, M.A. et al. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 163, 4833–4841 (1999).
Kwon, B.S. et al. Immune responses in 4-1BB (CD137)-deficient mice. J. Immunol. 168, 5483–5490 (2002).
Triantafilou, M. & Triantafilou, K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23, 301–304 (2002).
Han, J. & Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6, 1198–1205 (2005).
Hoebe, K. et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424, 743–748 (2003).
Kim, S.O., Ono, K., Tobias, P.S. & Han, J. Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J. Exp. Med. 197, 1441–1452 (2003).
Bukczynski, J., Wen, T., Ellefsen, K., Gauldie, J. & Watts, T.H. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 101, 1291–1296 (2004).
Zhou, H. et al. Determinants that control the specific interactions between TAB1 and p38α. Mol. Cell. Biol. 26, 3824–3834 (2006).
Han, J., Jiang, Y., Li, Z., Kravchenko, V.V. & Ulevitch, R.T. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386, 296–299 (1997).
Acknowledgements
We thank J. Peschon (Immunex), B. Beutler (The Scripps Research Institute) and S. Akira (Osaka University) for mice deficient in 4-1BBL, TRIF, MyD88, TLR4 and TLR2; and T. Wen (University of Toronto) for generating the mouse 4-1BBL adenovirus construct. Supported by the National Institutes of Health (GM67101, GM37696, AI41637 and AI54696 to J.H.; and EY13325, KRF-2005-084-E00001 and KRF-2005-201-E00008 to B.S.K.), the Canadian Institutes of Health (MT13220 to T.H.W., and MOP68841 to S.O.K.), the Science Research Center Fund from the Korea Science and Engineering Foundation (B.S.K.) and a Grant-in-Aid for the 21st Century Center of Excellence Program Topological Science and Technology from the Ministry of Education, Culture, Sport, Science, and Technology of Japan (S.S.).
Author information
Authors and Affiliations
Contributions
Y.J.K. designed the research, did most of the experiments, analyzed data and wrote the manuscript; S.O.K. and M.O. did experiments with macrophages from wild-type and 4-1BBL-deficient mice; S.S. did immunohistochemistry; A.S.-N. designed and generated '4-1BBL-knockdown' cell lines; T.H.W. and B.S.K. coordinated and organized the mouse experiments and contributed to the preparation of the manuscript; and J.H. designed the research, analyzed the data, supervised the experimental work and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
4-1BBL is required for sustained TNF production in RAW264.7 macrophages. (PDF 160 kb)
Supplementary Fig. 2
4-1BB is not involved in LPS-induced TNF production in RAW264.7 macrophages. (PDF 139 kb)
Supplementary Fig. 3
TLR ligands, but not IL-1β, induce 4-1BBL expression in macrophages. (PDF 444 kb)
Supplementary Fig. 4
4-1BBL is involved in TLR-ligands-induced TNF production in macrophages. (PDF 204 kb)
Supplementary Fig. 5
A model of the sequential signaling in LPS-treated macrophages. (PDF 112 kb)
Rights and permissions
About this article
Cite this article
Kang, Y., Kim, S., Shimada, S. et al. Cell surface 4-1BBL mediates sequential signaling pathways 'downstream' of TLR and is required for sustained TNF production in macrophages. Nat Immunol 8, 601–609 (2007). https://doi.org/10.1038/ni1471
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1471
This article is cited by
-
CD137L Inhibition Ameliorates Hippocampal Neuroinflammation and Behavioral Deficits in a Mouse Model of Sepsis-Associated Encephalopathy
NeuroMolecular Medicine (2023)
-
Resveratrol attenuates TLR-4 mediated inflammation and elicits therapeutic potential in models of sepsis
Scientific Reports (2020)
-
Crosstalk between signals initiated from TLR4 and cell surface BAFF results in synergistic induction of proinflammatory mediators in THP-1 cells
Scientific Reports (2017)
-
Transgenic 4-1BBL-engineered vaccine stimulates potent Gag-specific therapeutic and long-term immunity via increased priming of CD44+CD62Lhigh IL-7R+ CTLs with up- and downregulation of anti- and pro-apoptosis genes
Cellular & Molecular Immunology (2015)
-
MLK4 has negative effect on TLR4 signaling
Cellular & Molecular Immunology (2012)