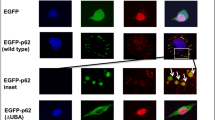
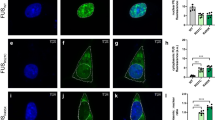
Abstract
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) have distinct clinical features but a common pathology—cytoplasmic inclusions rich in transactive response element DNA-binding protein of 43 kDa (TDP43). Rare TDP43 mutations cause ALS or FTD, but abnormal TDP43 levels and localization may cause disease even if TDP43 lacks a mutation. Here we show that individual neurons vary in their ability to clear TDP43 and are exquisitely sensitive to TDP43 levels. To measure TDP43 clearance, we developed and validated a single-cell optical method that overcomes the confounding effects of aggregation and toxicity and discovered that pathogenic mutations shorten TDP43 half-life. New compounds that stimulate autophagy improved TDP43 clearance and localization and enhanced survival in primary murine neurons and in human stem cell–derived neurons and astrocytes harboring mutant TDP43. These findings indicate that the levels and localization of TDP43 critically determine neurotoxicity and show that autophagy induction mitigates neurodegeneration by acting directly on TDP43 clearance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Robberecht, W. & Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 14, 248–264 (2013).
Neumann, M. Molecular neuropathology of TDP-43 proteinopathies. Int. J. Mol. Sci. 10, 232–246 (2009).
van Blitterswijk, M. & Landers, J.E. RNA processing pathways in amyotrophic lateral sclerosis. Neurogenetics 10.1007/s10048-010-0239-4 (2010).
Ayala, Y.M. et al. Structural determinants of the cellular localization and shuttling of TDP-43. J. Cell Sci. 121, 3778–3785 (2008).
Moisse, K. et al. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL−/− mice: support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 1296, 176–186 (2009).
Dewey, C.M. et al. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol. Cell. Biol. 31, 1098–1108 (2011).
Davidson, Y.S. et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's Syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 122, 703–713 (2011).
Barmada, S.J. & Finkbeiner, S. Pathogenic TARDBP mutations in amyotrophic lateral sclerosis and frontotemporal dementia: disease-associated pathways. Rev. Neurosci. 21, 251–272 (2010).
Barmada, S.J. et al. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J. Neurosci. 30, 639–649 (2010).
Tsao, W. et al. Rodent models of TDP-43: recent advances. Brain Res. 1462, 26–39 (2012).
Roberson, E.D. Mouse models of frontotemporal dementia. Ann. Neurol. 72, 837–849 (2012).
Ling, S.-C. et al. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc. Natl. Acad. Sci. USA 107, 13318–13323 (2010).
Watanabe, S., Kaneko, K. & Yamanaka, K. Accelerated disease onset with stabilized familial Amyotrophic Lateral Sclerosis (ALS)-linked TDP-43 mutations. J. Biol. Chem. 288, 3641–3654 (2013).
Roscic, A., Baldo, B., Crochemore, C., Marcellin, D. & Paganetti, P. Induction of autophagy with catalytic mTOR inhibitors reduces huntingtin aggregates in a neuronal cell model. J. Neurochem. 119, 398–407 (2011).
Prakash, A. & Levy, D.E. Regulation of IRF7 through cell type–specific protein stability. Biochem. Biophys. Res. Commun. 342, 50–56 (2006).
Wang, X. et al. Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci. Lett. 469, 112–116 (2010).
Urushitani, M., Sato, T., Bamba, H., Hisa, Y. & Tooyama, I. Synergistic effect between proteasome and autophagosome in the clearance of polyubiquitinated TDP-43. J. Neurosci. Res. 88, 784–797 (2010).
Zhang, X. et al. Rapamycin treatment augments motor neuron degeneration in SOD1G93A mouse model of amyotrophic lateral sclerosis. Autophagy 7, 412–425 (2011).
Hetz, C. et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 23, 2294–2306 (2009).
Wang, I.-F. et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc. Natl. Acad. Sci. USA 109, 15024–15029 (2012).
Fox, J.H. et al. The mTOR kinase inhibitor Everolimus decreases S6 kinase phosphorylation but fails to reduce mutant huntingtin levels in brain and is not neuroprotective in the R6/2 mouse model of Huntington's disease. Mol. Neurodegener. 5, 26 (2010).
Tsvetkov, A.S. et al. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc. Natl. Acad. Sci. USA 107, 16982–16987 (2010).
Laplante, M. & Sabatini, D.M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Gitcho, M.A. et al. TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. 63, 535–538 (2008).
Arrasate, M., Mitra, S., Schweitzer, E.S., Segal, M.R. & Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810 (2004).
Xu, Y.-F. et al. The pathological phenotypes of human TDP-43 transgenic mouse models are independent of downregulation of mouse Tdp-43. PLoS ONE 8, e69864 (2013).
Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat. Protoc. 2, 2024–2032 (2007).
Wong, E. & Cuervo, A.M. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb. Perspect. Biol. 2, a006734 (2010).
Klionsky, D.J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 (2008).
Wang, J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy 4, 947–948 (2008).
Kim, J., Dalton, V.M., Eggerton, K.P., Scott, S.V. & Klionsky, D.J. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol. Biol. Cell 10, 1337–1351 (1999).
Bilican, B. et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc. Natl. Acad. Sci. USA 109, 5803–5808 (2012).
Singh Roy, N. et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp. Neurol. 196, 224–234 (2005).
Geser, F. et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch. Neurol. 66, 180–189 (2009).
Kosik, K.S. & Finch, E.A. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J. Neurosci. 7, 3142–3153 (1987).
Boillée, S., Vande Velde, C. & Cleveland, D.W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 (2006).
Serio, A. et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl. Acad. Sci. USA 10.1073/pnas.1300398110 (2013).
Egawa, N. et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 5, 188lr2 (2012).
Stavrovskaya, I.G. Clinically approved heterocyclics act on a mitochondrial target and reduce stroke-induced pathology. J. Exp. Med. 200, 211–222 (2004).
Szczudlik, A., Tomik, B., Słowik, A. & Kasprzyk, K. Assessment of the efficacy of treatment with pimozide in patients with amyotrophic lateral sclerosis. Introductory notes. Neurol. Neurochir. Pol. 32, 821–829 (1998).
Krauss, S. et al. Translation of HTT mRNA with expanded CAG repeats is regulated by the MID1–PP2A protein complex. Nat. Commun. 4, 1511 (2013).
Miller, J. et al. Quantitative relationships between Huntingtin levels, polyglutamine length, inclusion body formation, and neuronal death provide novel insight into huntington's disease molecular pathogenesis. J. Neurosci. 30, 10541–10550 (2010).
Finkbeiner, S. Bridging the Valley of Death of therapeutics for neurodegeneration. Nat. Med. 16, 1227–1232 (2010).
Dolmetsch, R. & Geschwind, D.H. The Human brain in a dish: the promise of iPSC-derived neurons. Cell 145, 831–834 (2011).
Jacquier, A., Bellouze, S., Blanchard, S., Bohl, D. & Haase, G. Astrocytic protection of spinal motor neurons but not cortical neurons against loss of Als2/alsin function. Hum. Mol. Genet. 18, 2127–2139 (2009).
Haidet-Phillips, A.M. et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 (2011).
Ko, L.-W., Ko, H.-H.C., Lin, W.-L., Kulathingal, J.G. & Yen, S.-H.C. Aggregates assembled from overexpression of wild-type α-synuclein are not toxic to human neuronal cells. J. Neuropathol. Exp. Neurol. 67, 1084–1096 (2008).
Mucke, L. et al. High-level neuronal expression of aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Tsvetkov, A.S. et al. Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat. Chem. Biol. 9, 586–592 (2013).
Saudou, F., Finkbeiner, S., Devys, D. & Greenberg, M.E. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66 (1998).
Acknowledgements
We would like to thank B. Miller, B. Seeley, C. Lomen-Hoerth and the members of the Finkbeiner lab for their generous support and advice. H. Zahed and J. Margulis deserve special acknowledgment for their assistance. We thank G. Howard for editorial assistance and K. Nelson for administrative assistance. We also thank G. Yu and J. Herz (University of Texas, Southwestern) for anti-TDP43 antibodies. This work was supported by National Institutes of Neurological Disorders and Stroke grants K08 NS072233 (to S.J.B.), 3R01 NS039074, R01 NS083390, R43 NS081844 and U24 NS078370 (to S.F.); the ALS Association (S.F.); the Robert Packard Center for ALS Research and the William H. Adams Foundation (S.F.); and Target ALS (S.F.). Additional support was provided by the Roddenberry Stem Cell Program (to S.F.), the Taube-Koret Center for Neurodegenerative Disease (S.F.), the Hellman Family Foundation Alzheimer's Disease Research Program (S.F.), the Protein Folding Diseases Initiative at the University of Michigan (S.J.B.) and the California Institute of Regenerative Medicine TR4-06693 (S.F.) and U01 MH1050135 (S.F.). The animal care facility was partly supported by an US National Institutes of Health Extramural Research Facilities Improvement Program Project (C06 RR018928).
Author information
Authors and Affiliations
Contributions
S.J.B., A.S., A.A., A.T., S.C. and S.F. designed the study. S.J.B., A.A., X.L. and A.S. cultured cells, performed microscopy and conducted survival analyses. A.T., B.B., C.S. and S.C. provided compounds and iPSCs. M.P. performed in silico drug screening. D.P., D.M.A. and A.D. wrote original scripts for AFM. S.J.B. and A.A. analyzed data, constructed vectors, performed MPC and OPL and transfected neurons. S.B. and S.F. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–3 and Supplementary Figures 1–7. (PDF 2508 kb)
Rights and permissions
About this article
Cite this article
Barmada, S., Serio, A., Arjun, A. et al. Autophagy induction enhances TDP43 turnover and survival in neuronal ALS models. Nat Chem Biol 10, 677–685 (2014). https://doi.org/10.1038/nchembio.1563
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1563
This article is cited by
-
Janus kinase inhibitors are potential therapeutics for amyotrophic lateral sclerosis
Translational Neurodegeneration (2023)
-
Ubiquilin-2 regulates pathological alpha-synuclein
Scientific Reports (2023)
-
Amyotrophic lateral sclerosis: a neurodegenerative disorder poised for successful therapeutic translation
Nature Reviews Drug Discovery (2023)
-
Patients with sporadic FTLD exhibit similar increases in lysosomal proteins and storage material as patients with FTD due to GRN mutations
Acta Neuropathologica Communications (2023)
-
Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis
Nature Communications (2023)