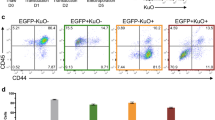
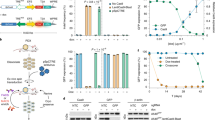
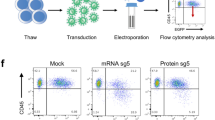
Abstract
Genome sequencing studies have shown that human malignancies often bear mutations in four or more driver genes1, but it is difficult to recapitulate this degree of genetic complexity in mouse models using conventional breeding. Here we use the CRISPR-Cas9 system of genome editing2,3,4 to overcome this limitation. By delivering combinations of small guide RNAs (sgRNAs) and Cas9 with a lentiviral vector, we modified up to five genes in a single mouse hematopoietic stem cell (HSC), leading to clonal outgrowth and myeloid malignancy. We thereby generated models of acute myeloid leukemia (AML) with cooperating mutations in genes encoding epigenetic modifiers, transcription factors and mediators of cytokine signaling, recapitulating the combinations of mutations observed in patients. Our results suggest that lentivirus-delivered sgRNA:Cas9 genome editing should be useful to engineer a broad array of in vivo cancer models that better reflect the complexity of human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Scuoppo, C. et al. A tumour suppressor network relying on the polyamine-hypusine axis. Nature 487, 244–248 (2012).
Zender, L. et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell 135, 852–864 (2008).
Hwang, W.Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Friedland, A.E. et al. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10, 741–743 (2013).
Shan, Q. et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31, 686–688 (2013).
Wood, A.J. et al. Targeted genome editing across species using ZFNs and TALENs. Science 333, 307 (2011).
Sander, J.D. et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 29, 697–698 (2011).
Hsu, P.D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Reyon, D. et al. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012).
Lee, B.H. et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell 12, 367–380 (2007).
Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826 (2013).
Kelly, L.M. & Gilliland, D.G. Genetics of myeloid leukemias. Annu. Rev. Genomics Hum. Genet. 3, 179–198 (2002).
Zhang, Y., Taylor, B.R., Shannon, K. & Clapp, D.W. Quantitative effects of Nf1 inactivation on in vivo hematopoiesis. J. Clin. Invest. 108, 709–715 (2001).
Tanaka, S. et al. Ezh2 augments leukemogenicity by reinforcing differentiation blockage in acute myeloid leukemia. Blood 120, 1107–1117 (2012).
Challen, G.A. et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 44, 23–31 (2012).
Moran-Crusio, K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24 (2011).
Quivoron, C. et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 20, 25–38 (2011).
Mead, A.J. et al. FLT3-ITDs instruct a myeloid differentiation and transformation bias in lymphomyeloid multipotent progenitors. Cell Reports 3, 1766–1776 (2013).
Schambach, A. et al. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol. Ther. 13, 391–400 (2006).
Kim, J.H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 6, e18556 (2011).
Morozova, K.S. et al. Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscopy. Biophys. J. 99, L13–L15 (2010).
Acknowledgements
The authors thank C. Morton and A. Hawkins from the Brigham and Women's Cytogenetics Core and R. Bronson from the DF/HCC Rodent Pathology Core for technical assistance and discussions. The authors thank F. Zhang for providing the CRISPR-Cas components. The authors also thank A. Schambach, E. Charpentier, J. Kroenke and A. Mullally for useful discussions, and thank S. Schwartz and B. Haas for assistance with analysis of sequencing data. C. Baum and A. Schambach of the Hannover Medical School, Hannover, Germany, kindly provided RRL.PPT.SFFV.IRES.eGFP.pre*, and D. Trono of EPFL, Lausanne, Switzerland, kindly provided both pMD2.G (Addgene plasmid 12259) and psPAX2 (Addgene plasmid 12260). This work was supported by funding from the National Institutes of Health (P01 CA108631), a Leukemia and Lymphoma Society Scholar Award, the SPARC consortium, a Center for Excellence in Genome Science grant (5P50HG006193-02 from the National Human Genome Research Institute) (A.R.) and Klarman Family Foundation at The Broad Institute (A.R.). D.H. was funded by the German Cancer Foundation (Mildred-Scheel Fellowship). M.S.K. is an EMBO and European Hematology Association Fellow.
Author information
Authors and Affiliations
Contributions
D.H., M.S.K. and B.L.E. designed experiments. D.H., M.S.K., D.Y., R.B., R.V.P., M.E.C. and A.T. performed the experiments. D.H., M.S.K., D.Y., R.B., J.C.A., A.R. and B.L.E. analyzed and interpreted the data. D.H. and B.L.E. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application relating to the work in this manuscript has been filed.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18, Supplementary Tables 1–4 and Supplementary Methods and Data (PDF 42106 kb)
Rights and permissions
About this article
Cite this article
Heckl, D., Kowalczyk, M., Yudovich, D. et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol 32, 941–946 (2014). https://doi.org/10.1038/nbt.2951
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2951
This article is cited by
-
Genome-scale pan-cancer interrogation of lncRNA dependencies using CasRx
Nature Methods (2024)
-
A potential paradigm in CRISPR/Cas systems delivery: at the crossroad of microalgal gene editing and algal-mediated nanoparticles
Journal of Nanobiotechnology (2023)
-
RNA binding protein IGF2BP1 synergizes with ETV6-RUNX1 to drive oncogenic signaling in B-cell Acute Lymphoblastic Leukemia
Journal of Experimental & Clinical Cancer Research (2023)
-
Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy
Molecular Cancer (2023)
-
OASL phase condensation induces amyloid-like fibrillation of RIPK3 to promote virus-induced necroptosis
Nature Cell Biology (2023)