Abstract
The prefrontal cortex is a critical neuroanatomical hub for controlling motivated behaviours across mammalian species1,2,3. In addition to intra-cortical connectivity, prefrontal projection neurons innervate subcortical structures that contribute to reward-seeking behaviours, such as the ventral striatum and midline thalamus4. While connectivity among these structures contributes to appetitive behaviours5,6,7,8,9,10,11,12,13, how projection-specific prefrontal neurons encode reward-relevant information to guide reward seeking is unknown. Here we use in vivo two-photon calcium imaging to monitor the activity of dorsomedial prefrontal neurons in mice during an appetitive Pavlovian conditioning task. At the population level, these neurons display diverse activity patterns during the presentation of reward-predictive cues. However, recordings from prefrontal neurons with resolved projection targets reveal that individual corticostriatal neurons show response tuning to reward-predictive cues, such that excitatory cue responses are amplified across learning. By contrast, corticothalamic neurons gradually develop new, primarily inhibitory responses to reward-predictive cues across learning. Furthermore, bidirectional optogenetic manipulation of these neurons reveals that stimulation of corticostriatal neurons promotes conditioned reward-seeking behaviour after learning, while activity in corticothalamic neurons suppresses both the acquisition and expression of conditioned reward seeking. These data show how prefrontal circuitry can dynamically control reward-seeking behaviour through the opposing activities of projection-specific cell populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Langworthy, O. R. Increased spontaneous activity produced by frontal lobe lesion in cats. Am. J. Physiol. 126, 158–161 (1939)
Matsumoto, K., Suzuki, W. & Tanaka, K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science 301, 229–232 (2003)
Warden, M. R. et al. A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432 (2012)
Vertes, R. P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58 (2004)
Britt, J. P. et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012)
Ma, Y. Y. et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83, 1453–1467 (2014)
McFarland, K. & Kalivas, P. W. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 21, 8655–8663 (2001)
McFarland, K., Lapish, C. C. & Kalivas, P. W. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 23, 3531–3537 (2003)
Neumann, P. A. et al. Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology 41, 2399–2410 (2016)
Pascoli, V. et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464 (2014)
Peters, J., LaLumiere, R. T. & Kalivas, P. W. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci. 28, 6046–6053 (2008)
Stefanik, M. T. et al. Optogenetic inhibition of cocaine seeking in rats. Addict. Biol. 18, 50–53 (2013)
Ye, L. et al. Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell 165, 1776–1788 (2016)
Bouret, S. & Sara, S. J. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur. J. Neurosci. 20, 791–802 (2004)
Kim, H., Ährlund-Richter, S., Wang, X., Deisseroth, K. & Carlén, M. Prefrontal parvalbumin neurons in control of attention. Cell 164, 208–218 (2016)
Kobayashi, S. et al. Influences of rewarding and aversive outcomes on activity in macaque lateral prefrontal cortex. Neuron 51, 861–870 (2006)
Moorman, D. E. & Aston-Jones, G. Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc. Natl Acad. Sci. USA 112, 9472–9477 (2015)
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013)
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl Acad. Sci. USA 101, 18206–18211 (2004)
Sesack, S. R., Deutch, A. Y., Roth, R. H. & Bunney, B. S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213–242 (1989)
Bossert, J. M. et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J. Neurosci. 32, 4982–4991 (2012)
McGlinchey, E. M., James, M. H., Mahler, S. V., Pantazis, C. & Aston-Jones, G. Prelimbic to accumbens core pathway is recruited in a dopamine-dependent manner to drive cued reinstatement of cocaine seeking. J. Neurosci. 36, 8700–8711 (2016)
Igelstrom, K. M., Herbison, A. E. & Hyland, B. I. Enhanced c-Fos expression in superior colliculus, paraventricular thalamus and septum during learning of cue-reward association. Neuroscience 168, 706–714 (2010)
Haight, J. L. & Flagel, S. B. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front. Behav. Neurosci. 8, 79 (2014)
Do-Monte, F. H., Quiñones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015)
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015)
Harris, K. D. & Mrsic-Flogel, T. D. Cortical connectivity and sensory coding. Nature 503, 51–58 (2013)
Pinto, A. & Sesack, S. R. Limited collateralization of neurons in the rat prefrontal cortex that project to the nucleus accumbens. Neuroscience 97, 635–642 (2000)
Resendez, S. L. et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat. Protocols 11, 566–597 (2016)
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protocols 7, 12–23 (2011)
Soudais, C., Laplace-Builhe, C., Kissa, K. & Kremer, E. J. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15, 2283–2285 (2001)
Hnasko, T. S. et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc. Natl Acad. Sci. USA 103, 8858–8863 (2006)
Kaifosh, P., Zaremba, J. D., Danielson, N. B. & Losonczy, A. SIMA: Python software for analysis of dynamic fluorescence imaging data. Front. Neuroinform. 8, 80 (2014)
Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. IJCAI (U. S.) 95, 1137–1143 (1995)
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011)
Conte, W. L., Kamishina, H. & Reep, R. L. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat. Protocols 4, 1157–1166 (2009)
Callaway, E. M. Transneuronal circuit tracing with neurotropic viruses. Curr. Opin. Neurobiol. 18, 617–623 (2008)
Hoover, W. B. & Vertes, R. P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179 (2007)
Otis, J. M., Dashew, K. B. & Mueller, D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J. Neurosci. 33, 1271–1281 (2013)
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotactic Coordinates 3rd edn (Academic Press, 2007)
Acknowledgements
We thank S. Smith and J. Stirman for helpful discussions. This study was funded by grants from the National Institutes of Health (NIDA: F32-DA041184, J.M.O.; R01-DA032750, G.D.S.; R01-DA038168, G.D.S.; NICHD: T32-HD079124, S.L.R.; NIMH: T32-MH093315, J.A.M.), the Brain and Behavior Research Foundation (G.D.S.), the Children’s Tumor Foundation (016-01-006, J.E.R.), the Foundation of Hope (G.D.S.), the UNC Neuroscience Center (Helen Lyng White Fellowship, V.M.K.N.), the UNC Neuroscience Center Microscopy Core (P30 NS045892), and the UNC Department of Psychiatry (G.D.S.).
Author information
Authors and Affiliations
Contributions
V.M.K.N. wrote codes for analyses. V.M.K.N., A.M.M., E.S.V., E.P.M., O.K., J.A.M., J.E.R., S.L.R. and M.A.R. provided technical assistance for in vivo optogenetics, histology and immunohistochemistry. J.M.O. performed experiments and surgeries. J.M.O., V.M.K.N. and G.D.S. designed the experiments, analysed and interpreted the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Mice used for imaging experiments acquired cue-specific anticipatory licking across conditioning.
a, Average lick rate during the 1-s baseline period (immediately before each cue delivery) for all imaging experiments (early, n = 30 sessions; late n = 30 sessions). b, Average lick rate during each cue (rather than the change in lick rate presented in the main figures) for all imaging experiments (early, n = 30 sessions; late n = 30 sessions). c, Individual behavioural discrimination (licking during CS+ versus CS−; auROC − 0.5) scores during early and late conditioning sessions for all imaging sessions used in this manuscript (early, n = 30 sessions; late, n = 30 sessions; t58 = 17.6, P < 0.001). Line graphs represent the mean ± s.e.m. These data are presented in a summarized form in Fig. 1d, e.
Extended Data Figure 2 Elevations and reductions in GCaMP6s fluorescence track action potential frequency but not voltage per se.
a, Virus injections of AAVdj-CaMK2a-GCaMP6s into dorsomedial PFC allowed subsequent whole-cell patch-clamp slice recordings of GCaMP6s-expressing neurons. Coronal cartoon redrawn based on ref. 40. b, c, Example traces showing GCaMP6s fluorescence (b) during current-clamp recordings (c) in the absence (left) and presence (right) of action potentials (n = 8 neurons; n = 2 mice). d, Grouped data revealing that hyperpolarization resulted in negative GCaMP6s fluorescence deflections in the presence of baseline action potentials, but not in the absence of baseline action potentials (interaction: F1,14 = 20.0; ***P < 0.001; post hoc tests: baseline action potentials, P > 0.4; no baseline action potentials, P < 0.001). e, Example traces showing a series of depolarizing pulses (1–20 Hz) applied in current-clamp mode to drive trains of action potentials (bottom), during which GCaMP6s fluorescence was tracked in recorded neurons (top; n = 12 neurons; n = 2 mice). f, Action potential generation resulted in linear elevations in GCaMP6s fluorescence (r = 0.776, ***P < 0.001), such that a single action potential was detectable (red waveform; peak = 12.6 ± 4.0% Δf/f0). g, A series of baseline depolarizing pulses (1–20Hz) was applied before and after a 3 s pause (n = 7 neurons; n = 2 mice). h, The pause in action potentials resulted in linear reductions in GCaMP6s fluorescence (r = −0.656, ***P < 0.001), such that a 1 Hz reduction in firing frequency was detectable (red waveform; peak = −8.4 ± 2.0% Δf/f0). Line graphs represent the mean ± s.e.m. AP, action potential; ILc, infralimbic cortex; PLc, prelimbic cortex.
Extended Data Figure 3 Cue responses in PFC CaMK2a-expressing neurons, PFC–NAc neurons, and PFC–PVT neurons before appetitive learning.
a, b, Population heat plots showing average responses for all individual PFC CaMK2a-expressing neurons (n = 1,473 neurons; n = 8 mice) across all CS− trials before learning (a) and all CS+ trials before learning (b). c, d, Population heat plots showing average responses for all individual PFC–NAc neurons (n = 84 neurons; n = 4 mice) across all CS− trials before learning (c) and all CS+ trials before learning (d). e, f, Population heat plots showing average responses for all individual PFC–PVT neurons (n = 92 neurons; n = 3 mice) across all CS− trials before learning (e) and all CS+ trials before learning (f). Data shown here are from neurons presented in Figs 1 and 2. Vertical dotted lines refer to the time of sucrose delivery in CS+ trials or the equivalent time for CS− trials.
Extended Data Figure 4 Cue discrimination in PFC CaMK2a-expressing neurons before and after appetitive learning.
a–c, Histograms for all recorded CaMK2a-expressing PFC neurons (early, n = 1,473 neurons; late, n = 1,569 neurons; n = 8 mice), showing CS+ responses (a), CS− responses (b), and CS+/CS− discrimination (c) during both early and late Pavlovian conditioning sessions. d, CDF plot showing that the dynamics of individual CaMK2a-expressing neurons could be used to accurately decode whether the CS+ or CS− was presented in early conditioning sessions (compared to early shuffled: Welch’s t2,925.61 = 7.30, P < 0.001), as well as in late conditioning sessions (compared to late shuffled: Welch’s t2,727.06 = 24.84, P < 0.001). Data shown here are from neurons presented in Fig. 1. EarlySh, early shuffled; LateSh, late shuffled.
Extended Data Figure 5 Corticostriatal and corticothalamic projection neurons are anatomically and electrophysiologically distinct.
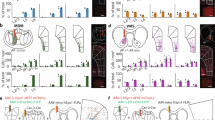
a, b, CtB-488 and CtB-594 were injected (n = 3 mice) into either NAc or PVT (scale bars, 250 μm) (a), resulting in retrograde labelling of NAc-projecting and PVT-projecting neurons in dorsomedial PFC (scale bars, 50 μm) (b). c–e, Coronal sections of anterior (c), middle (d), and posterior (e) dorsomedial PFC revealing spatial separation of PFC–NAc and PFC–PVT neurons (scale bars, 250 μm). f–h, Population histograms from all mice (n = 3) showing cell counts from anterior (f), middle (g), and posterior (h) dorsomedial PFC reveal that NAc-projecting neurons (red) are in more superficial layers as compared to PVT-projecting neurons (blue). Black refers to overlap (medial–lateral axis) between red and blue bars, and purple refers to double-labelled neurons (that is, both NAc- and PVT-projection neurons). i, Current-clamp recordings from dorsomedial PFC CtB-labelled neurons projecting to either NAc (top; n = 9 neurons; n = 3 mice; scale bars = 25 μm) or PVT (bottom; n = 10 neurons; n = 3 mice; scale bars = 25 μm). j, k, Representative waveforms (j) and averaged data (k) showing that PFC–NAc neurons fired fewer action potentials (spikes) as compared to PFC–PVT neurons during somatic depolarization (interaction: F16,272 = 16.6, ***P < 0.001). l, m, Representative waveforms (l) and averaged data (m) revealing no differences in rheobase (the minimum current required to evoke an action potential) between PFC–NAc and PFC–PVT neurons (t17 = 1.22, P > 0.2 (n.s., not significant)). n, o, Representative waveforms (n) and averaged data (o) showing that PFC–NAc neurons had larger peak afterhyperpolarizations (AHPs) as compared to PFC–PVT neurons (t17 = 4.67, ***P < 0.001). p, The maximum number of action potentials (spikes) in each neuron was correlated with the peak afterhyperpolarization (r = 0.80, P < 0.0001). Line and bar graphs represent the mean ± s.e.m. cc, corpus callosum; ILc, infralimbic cortex; MO, medial orbitofrontal cortex; PLc, prelimbic cortex; VO ventral orbitofrontal cortex.
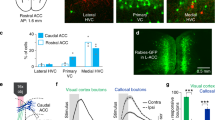
Extended Data Figure 6 Corticostriatal and corticothalamic projection neurons have distinct monosynaptic inputs.
a–d, Viral strategy for rabies tracing experiments in which the monosynaptic inputs to PFC–PVT (a, b) and PFC–NAc (c, d) neurons were evaluated (n = 3 mice per group). e–g, Example images showing mCherry+ cells (TVA expression) (e), RV-GFP+ (rabies virus encoding GFP) cells (f), and overlap revealing mCherry+/RV-GFP+ cells (starter cells; mCherry−/RV-GFP+ cells are considered local inputs) (g). h, The number of local input neurons (nonstarter; only RV-GFP+ cells per section) to each projection population, as quantified by raw neuron count and by the per cent of starter cells for each mouse, was equivalent for PFC–NAc and PFC–PVT neurons (raw neuron count: t16 = 0.59, P = 0.56; per cent starter cells: t16 = 0.13, P = 0.90). i, Representative image showing RV-GFP but not mCherry expression in the anterior cingulate cortex (ACC). j, The number of input neurons from ACC was higher for PFC–PVT neurons as compared to PFC–NAc neurons (raw neuron count: t16 = 3.51; **P = 0.003; per cent starter cells: t16 = 3.31, **P = 0.004). k, Representative image showing RV-GFP but not mCherry expression in the lateral pre-optic area (LPO). l, The number of input neurons from the LPO was equivalent for PFC–NAc and PFC–PVT cells (raw neuron count: t16 = 1.77; P = 0.10; per cent starter cells: t16 = 0.20, P = 0.84). m, Representative image showing RV-GFP but not mCherry expression in the ventral hippocampus (vHipp). n, The number of input neurons from the ventral hippocampus was higher for PFC–NAc neurons as compared with PFC–PVT neurons (raw neuron count: t16 = 4.44; ***P < 0.001; per cent starter cells: t16 = 4.00, **P = 0.001). o, Representative image showing RV-GFP but not mCherry expression in the ventral tegmental area (VTA). p, The number of input neurons from the VTA was equivalent for PFC–NAc and PFC–PVT cells (raw neuron count: t16 = 0.56; P = 0.59; per cent starter cells: t16 = 0.09, P = 0.93). Bar graphs represent the mean ± s.e.m. Note that no RV-GFP+ neurons were detected in any nucleus of the amygdala for either projection group.
Extended Data Figure 7 Corticostriatal and corticothalamic projection neurons express CaMK2a and have distinct basal activity dynamics.
a–d, Injections of AAV5-CaMK2a-eYFP into dorsomedial PFC and the retrograde tracer CtB-594 into NAc (a) or PVT (c) resulted in expression of eYFP in CtB-labelled PFC–NAc neurons (b) and PFC–PVT neurons (d). These data reveal that PFC–NAc and PFC–PVT are subpopulations of CaMK2a-expressing neurons (n = 2 mice per group). e, f, In ai9 reporter mice, (e) AAVdj-DIO-GCaMP6s injections in dorsomedial PFC and Cav2-Cre injections into PVT (f) result in expression of GCaMP6s and tdTomato (marker for Cre-recombinase), which have spatial overlap in PFC (n = 2 mice). These data reveal that GCaMP6s expression is specific to the projection cells of interest. g, Example traces revealing spontaneous calcium dynamics from in vivo two-photon imaging in GCaMP6s-expressing PFC–NAc neurons (top; n = 69 neurons; n = 4 mice) and PFC–PVT neurons (bottom; n = 63; n = 3 mice) in awake, head-fixed mice. Red and blue dots refer to detected events. h–j, Averaged data reveal no differences in event amplitude (h) or event duration (i); however, PFC–NAc neurons had significantly shorter inter-event intervals (j) as compared to PFC–PVT neurons (amplitude: t130 = 1.10, P > 0.2; duration: t130 = 0.68, P > 0.4; interval: t130 = 2.30, *P < 0.05). Bar graphs represent the mean ± s.e.m.
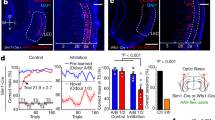
Extended Data Figure 8 Effects of corticostriatal and corticothalamic optogenetic manipulations on acquisition and expression of CS− licking.
a, Line graph showing average CS− lick rate during conditioning (with laser) and test (no laser) from PFC–NAc::ChR2 (n = 5), PFC–NAc::eNpHR3.0 (n = 6), and PFC–NAc::eYFP mice (n = 10). b, c, CDF plots and bar graphs showing CS− lick rate during conditioning (b) and test (c). No differences were observed between PFC–NAc groups during the no-laser test (ChR2 versus eYFP: auROC = 0.47, Benjamini–Hochberg corrected P = 0.45; eNpHR3.0 versus eYFP: auROC = 0.47, P = 0.45). d, Line graph showing average CS− lick rate during conditioning (with laser) and test (no laser) from PFC–PVT::ChR2 (n = 6), PFC–NAc::PVT (n = 5), and PFC–PVT::eYFP mice (n = 10). e, f, CDF plots and bar graphs showing CS− lick rate during conditioning (e) and test (f). No differences were observed between PFC–PVT groups during the no-laser test (ChR2 versus eYFP: auROC = 0.53, Benjamini–Hochberg corrected P = 0.44; eNpHR3.0 versus eYFP: auROC = 0.35, P = 0.36). g–i, CDF plots and bar graphs showing CS− lick rates for PFC–NAc::ChR2 (n = 5), PFC–NAc::eNpHR3.0 (n = 5), and PFC–NAc::eYFP mice (n = 6). There were no significant differences in CS− lick rate for PFC–NAc::ChR2 mice (versus PFC–NAc::eYFP: auROC = 0.43, P = 0.26), although there was an effect of laser for PFC–NAc::eNpHR3.0 mice (versus PFC–NAc::eYFP: auROC = 0.23, *P = 0.006). j–l, CDF plots and bar graphs showing CS− lick rates for PFC–PVT::ChR2 (n = 5), PFC–PVT::eNpHR3.0 (n = 5), and PFC–PVT::eYFP mice (n = 8). There were no significant differences in CS− lick rate for PFC–PVT::ChR2 mice (versus PFC–PVT::eYFP: auROC = 0.35, P = 0.15) or PFC–PVT::eNpHR3.0 mice (versus PFC–PVT::eYFP: auROC = 0.55, P = 0.31). Line and bar graphs represent the mean ± s.e.m. NL, no-laser test.
Extended Data Figure 9 Effects of corticostriatal and corticothalamic optogenetic manipulations are timing dependent.
a–c, CDF plots (top) and bar graphs (bottom) show anticipatory licking rates for PFC–NAc::ChR2 (n = 5) or PFC–NAc::eNpHR3.0 (n = 5) versus PFC–NAc::eYFP mice (n = 6) during sessions in which the laser was randomly presented outside of cue delivery. There were no significant differences in anticipatory lick rate for PFC–NAc::ChR2 mice (versus PFC–NAc::eYFP: auROC = 0.56, Benjamini–Hochberg corrected P = 0.30) or PFC–NAc::eNpHR3.0 mice (versus PFC–NAc::eYFP: auROC = 0.63, P = 0.23). d–f, CDF plots (top) and bar graphs (bottom) show anticipatory licking rates for PFC–PVT::ChR2 (n = 5) or PFC–PVT::eNpHR3.0 (n = 5) versus PFC–PVT::eYFP (n = 8) mice during sessions in which the laser was randomly presented outside of cue delivery. There were no significant differences in anticipatory lick rate for PFC–PVT::ChR2 mice (versus PFC–PVT::eYFP: auROC = 0.42, P = 0.21) or PFC–PVT::eNpHR3.0 mice (versus PFC–PVT::eYFP: auROC = 0.36, P = 0.14). g–i, CDF plots (top) and bar graphs (bottom) show CS− lick rates for PFC–NAc::ChR2 (n = 5) or PFC–NAc::eNpHR3.0 (n = 5) versus PFC–NAc::eYFP mice (n = 6) during sessions in which the laser was randomly presented outside of cue delivery. There were no significant differences in CS− lick rate for PFC–NAc::ChR2 mice (versus PFC–NAc::eYFP: auROC = 0.41, P = 0.19) or PFC–NAc::eNpHR3.0 mice (versus PFC–NAc::eYFP: auROC = 0.40, P = 0.19). j–l, CDF plots (top) and bar graphs (bottom) show CS− lick rates for PFC–PVT::ChR2 (n = 5) or PFC–PVT::eNpHR3.0 (n = 5) versus PFC–PVT::eYFP (n = 8) mice during sessions in which the laser was randomly presented outside of cue delivery. There were no significant differences in CS− lick rate for PFC–PVT::ChR2 mice (versus PFC–PVT::eYFP: auROC = 0.39, P = 0.12) or PFC–PVT::eNpHR3.0 mice (versus PFC–PVT::eYFP: auROC = 0.36, P = 0.12). Bar graphs represent the mean ± s.e.m.
Extended Data Figure 10 Optogenetic manipulations of corticostriatal and corticothalamic neurons are not appetitive, aversive, and do not affect movement.
Mice underwent a freely moving real-time place preference assay for 20 minutes to assess the rewarding or aversive value of PFC–NAc or PFC–PVT stimulation or inhibition (laser and group parameters are identical to head-fixed optogenetics experiments; see Methods). a, Tracking data from single example mice showing that PFC–NAc::ChR2 (left; n = 5) and PFC–NAc::eNpHR3.0 (right; n = 5) mice spent equivalent time in chambers that were paired with laser (PFC–NAc::eYFP mice, n = 8). b, Grouped data show that laser stimulation in PFC–NAc mice did not lead to a real-time place preference (interaction: F2,30 = 2.15, P > 0.13). c, Grouped data show that optogenetic manipulations in PFC–NAc mice did not influence velocity of movement (interaction: F2,30 = 0.12, P > 0.88). d, Tracking data from single example mice during real-time place preference experiments showing that PFC–PVT::ChR2 (left; n = 5) and PFC–PVT::eNpHR3.0 (right; n = 5) mice spent equivalent time in chambers that were paired with laser (PFC–PVT::eYFP mice, n = 5). e, Grouped data show that laser stimulation in PFC–PVT mice did not lead to a real-time place preference (interaction: F2,24 = 0.15, P > 0.86). f, Grouped data show that optogenetic stimulation in PFC–PVT did not influence velocity of movement (interaction: F2,24 = 0.10, P > 0.90). g, h, Coronal plates (redrawn from ref. 40) located 1.98, 1.78, and 1.54 mm anterior to bregma illustrate the placements of optical fibre tips in PFC–NAc mice (g) and PFC–PVT mice (h). Bar graphs represent the mean ± s.e.m. NoStim, no laser stimulation; Stim, laser stimulation.
Supplementary information
Representative video revealing calcium dynamics of dorsal medial PFC GCaMP6s-expressing neurons
Injection of AAV-CaMKII-GCaMP6s resulted in dynamic GCaMP6s fluorescence from hundreds of visible PFC neurons in vivo (see methods for details). Data acquisition occurred at 2.5Hz, and this representative video is shown at 10X normal speed (25 frames per second; 485 x 508 pixels; 373μmß x 390μm). (MOV 19846 kb)
Rights and permissions
About this article
Cite this article
Otis, J., Namboodiri, V., Matan, A. et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 543, 103–107 (2017). https://doi.org/10.1038/nature21376
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21376
This article is cited by
-
A prefrontal-thalamic circuit encodes social information for social recognition
Nature Communications (2024)
-
Frontostriatal circuit dysfunction leads to cognitive inflexibility in neuroligin-3 R451C knockin mice
Molecular Psychiatry (2024)
-
Determinants of functional synaptic connectivity among amygdala-projecting prefrontal cortical neurons in male mice
Nature Communications (2023)
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression
Neuropsychopharmacology (2023)
-
The claustrum-prelimbic cortex circuit through dynorphin/κ-opioid receptor signaling underlies depression-like behaviors associated with social stress etiology
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.