Abstract
Thirst is the basic instinct to drink water. Previously, it was shown that neurons in several circumventricular organs of the hypothalamus are activated by thirst-inducing conditions1. Here we identify two distinct, genetically separable neural populations in the subfornical organ that trigger or suppress thirst. We show that optogenetic activation of subfornical organ excitatory neurons, marked by the expression of the transcription factor ETV-1, evokes intense drinking behaviour, and does so even in fully water-satiated animals. The light-induced response is highly specific for water, immediate and strictly locked to the laser stimulus. In contrast, activation of a second population of subfornical organ neurons, marked by expression of the vesicular GABA transporter VGAT, drastically suppresses drinking, even in water-craving thirsty animals. These results reveal an innate brain circuit that can turn an animal’s water-drinking behaviour on and off, and probably functions as a centre for thirst control in the mammalian brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McKinley, M. J. et al. The sensory circumventricular organs of the mammalian brain. Adv. Anat. Embryol. Cell Biol. 172, 1–122 (2003)
Young, J. K. Hunger, Thirst, Sex, and Sleep: How the Brain Controls Our Passions (Rowman & Littlefield, 2012)
Sternson, S. M. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824 (2013)
Daniels, D. & Fluharty, S. J. Salt appetite: a neurohormonal viewpoint. Physiol. Behav. 81, 319–337 (2004)
Geerling, J. C. & Loewy, A. D. Central regulation of sodium appetite. Exp. Physiol. 93, 177–209 (2008)
Oka, Y., Butnaru, M., von Buchholtz, L., Ryba, N. J. & Zuker, C. S. High salt recruits aversive taste pathways. Nature 494, 472–475 (2013)
Stricker, E. M. & Sved, A. F. Thirst. Nutrition 16, 821–826 (2000)
McKinley, M. J. & Johnson, A. K. The physiological regulation of thirst and fluid intake. News Physiol. Sci. 19, 1–6 (2004)
Fitzsimons, J. T. Angiotensin, thirst, and sodium appetite. Physiol. Rev. 78, 583–686 (1998)
Epstein, A. N., Fitzsimons, J. T. & Simons, B. J. Drinking caused by the intracranial injection of angiotensin into the rat. J. Physiol. (Lond.) 200, 98–100 (1969)
Sturgeon, R. D., Brophy, P. D. & Levitt, R. A. Drinking elicited by intracranial microinjection of angiotensin in the cat. Pharmacol. Biochem. Behav. 1, 353–355 (1973)
Robertson, A., Kucharczyk, J. & Mogenson, G. J. Drinking behavior following electrical stimulation of the subfornical organ in the rat. Brain Res. 274, 197–200 (1983)
Smith, P. M., Beninger, R. J. & Ferguson, A. V. Subfornical organ stimulation elicits drinking. Brain Res. Bull. 38, 209–213 (1995)
Verbalis, J. G. How does the brain sense osmolality? J. Am. Soc. Nephrol. 18, 3056–3059 (2007)
Bourque, C. W., Oliet, S. H. & Richard, D. Osmoreceptors, osmoreception, and osmoregulation. Front. Neuroendocrinol. 15, 231–274 (1994)
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl Acad. Sci. USA 102, 17816–17821 (2005)
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005)
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011)
Feil, R. et al. Ligand-activated site-specific recombination in mice. Proc. Natl Acad. Sci. USA 93, 10887–10890 (1996)
Hiyama, T. Y., Watanabe, E., Okado, H. & Noda, M. The subfornical organ is the primary locus of sodium-level sensing by Nax sodium channels for the control of salt-intake behavior. J. Neurosci. 24, 9276–9281 (2004)
Noda, M. & Sakuta, H. Central regulation of body-fluid homeostasis. Trends Neurosci. 36, 661–673 (2013)
Johnson, A. K., Zardetto-Smith, A. M. & Edwards, G. L. Integrative mechanisms and the maintenance of cardiovascular and body fluid homeostasis: the central processing of sensory input derived from the circumventricular organs of the lamina terminalis. Prog. Brain Res. 91, 381–393 (1992)
Johnson, A. K. & Gross, P. M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 7, 678–686 (1993)
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 10.1016/j.neuron.2011.05.028. (2011)
Garcia, A. D., Doan, N. B., Imura, T., Bush, T. G. & Sofroniew, M. V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature Neurosci. 7, 1233–1241 (2004)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 13, 133–140 (2010)
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M. & Dulac, C. G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330 (2014)
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neurosci. 14, 351–355 (2011)
Lee, H. et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014)
Chandrashekar, J. et al. The cells and peripheral representation of sodium taste in mice. Nature 464, 297–301 (2010)
Ng, L, et al. An anatomic gene expression atlas of the adult mouse brain. Nature Neurosci. 12, 356–362 (2009)
Yoneshima, H, et al. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience 137, 401–412 (2006)
Acknowledgements
We thank N. Propp for help with mouse husbandry. We also thank H. Fishman for suggestions, Z. Turan, N. Ryba and T. Usdin for technical support, and N. Ryba and members of the Zuker laboratory for comments. We acknowledge B. Lowell and M. Krashes for advice. Y.O. and M.Y. were supported by grants from the National Institute on Drug Abuse and National Institute of Neurological Disorders and Stroke to C.S.Z. C.S.Z. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Y.O. developed the research program, designed the study, carried out the experiments, and analysed data; M.Y. performed all slice patch clamp recordings; C.S.Z. analysed data, designed experiments and together with Y.O. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Fos induction by water deprivation in the SFO.
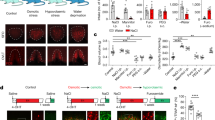
a, Approximately 30% of cells in the SFO (visualized with 4′,6-diamidino-2-phenylindole (DAPI) staining, blue) of a water-restricted animal (48 h) are Fos-positive (red, 26 ± 1.9%, n = 3). b, No significant Fos labelling was detected under the water-satiated condition (lower panel). Scale bar, 50 μm. The graph shows quantification of Fos-positive cells among CamKII+ neurons, both under water-restricted and satiated conditions. Values are means ± s.e.m. (n = 3).
Extended Data Figure 2 nNOS-positive neurons in the SFO co-express Vglut2, an excitatory neuronal marker.
nNOS antibody staining (green) of the SFO from a Slc17a6-Cre/Ai9 transgenic animal expressing tdTomato in Vglut2-positive neurons (red); the right panel shows a magnified view illustrating the overlap between tdTomato- and nNOS-positive signals. Scale bar, 50 μm.
Extended Data Figure 3 Virally expressed ChR2–eYFP under the control of CamKII promoter co-localized with endogenous CamKII.
Tissue staining of the SFO in a wild-type animal infected with AAV-CamKIIa-ChR2–eYFP. Expression of ChR2–eYFP (labelled with anti-GFP antibody) overlapped endogenous CamKII expression (anti-CamKII antibody, upper right). Lower left panel shows DAPI staining (blue). Scale bar, 50 μm.
Extended Data Figure 4 ChR2-dependent drinking requires activation of SFO CamKII-positive neurons concurrently with water presentation.
a, Representative raster plots illustrating drinking behaviour in a wild-type animal expressing ChR2–eYFP under the control of CamKII promoter. Trials were performed with photostimulation (blue shadings) delivered before (trials 1–5) or during (trials 6–10) water presentation. The filled arrowhead indicates the time of water presentation in each trial. Each black bar denotes an individual licking event. Note that photostimulation in the absence of water does not lead to drinking after stimulation, even if water is presented a few seconds after the termination of the light stimulation. b, Quantification of drinking responses in six animals expressing AAV-flex-ChR2–eYFP in CamKII-positive neurons before (red bar) and during (black bar) water presentation (Mann–Whitney U-test, P < 0.003). Animals were tested for five trials each, and the total number of licks was averaged across trials. Values are means ± s.e.m.
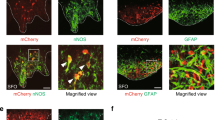
Extended Data Figure 5 Three distinct cell populations in the SFO.
Using a combination of data from the Allen Brain Atlas (http://mouse.brain-map.org)31 and a candidate gene approach, we identified three genetically separable, non-overlapping populations in the SFO. One population is defined by the expression of nNOS (red); also overlapping with CamKII- and ETV-1-positive cells (see Fig. 3a, b). A second is a Vgat-expressing population visualized in a transgenic animal expressing ChR2–eYFP in Vgat-positive neurons (labelled with anti-GFP antibody, green). A third is characterized by the expression of GFAP (white). Shown are double immunohistochemical stainings of nNOS- and Vgat-positive neurons (top panel), nNOS- and GFAP-positive neurons (middle panel), and GFAP- and Vgat-positive neurons (bottom panel). Scale bar, 50 μm.
Extended Data Figure 6 Angiotensin receptor AT1 is enriched in ETV-1+ neurons in the SFO.
Quantitative PCR analysis of gene expression in three groups of neurons: ETV-1+ neurons from the SFO, ETV-1+ from the cerebral cortex32, and Vgat+ neurons from the SFO. Individual data points were normalized to the levels of GAPDH. Shown are the quantitative PCR results for ETV1, nNOS, Vgat and AT1 in the three different samples; data are presented for each gene as its relative abundance compared with the tissue with the lowest level of expression (for example nNOS is expressed ten times more abundantly in ETV-1-positive neurons from the cortex than in Vgat neurons from the SFO, and 1,000 times more abundantly in ETV-1-positive neurons from the SFO than the cortex). Note that AT1 is highly enriched in ETV-1+ SFO neurons. ND, not detected. Values are means ± s.e.m. (n = 3 technical replicates).
Extended Data Figure 7 Neural projections from Vgat- and Etv-1-positive SFO neurons.
Slc32a1-Cre (Vgat-cre; left panel) and Etv1-CreER (right panel) mice were independently injected with AAV-flex-tdTomato in the SFO, and the axon projections of Vgat-positive and ETV1-positive neurons examined using tdTomato reporter expression (red). Shown are the injection sites (top panels) and representative images of four brain regions receiving input from the SFO: OVLT, organum vasculosum of the lamina terminalis; MnPO, median preoptic nucleus; SO, supraoptic nucleus; PVN, paraventricular hypothalamic nucleus. Although we cannot preclude additional sites, these four areas exhibited the most prominently labelled projections from the SFO neurons. Scale bars, 200 μm.
Supplementary information
Stimulation of CamKII-positive Neurons in the SFO Immediately Triggers Drinking Behaviour
A water-satiated animal expressing ChR2-EYFP under the control of CamKII promoter was photostimulated as shown (“Light”). Upon photostimulation, the animal immediately ceased current activities, searched for water, and started drinking. Note that the animal quickly stopped drinking upon termination of photostimulation. (WMV 9130 kb)
Stimulation of CamKII-positive Neurons in the SFO Triggers Drinking
Upon photostimulation, mice display robust ChR2-dependent drinking, even if water was presented in an (unfamiliar) object that the animal has never encountered (white bowl). (WMV 3804 kb)
Stimulation of SFO neurons does not induce feeding
Photostimulation (indicated by “Light”) did not trigger simultaneous feeding responses. (WMV 3088 kb)
Rights and permissions
About this article
Cite this article
Oka, Y., Ye, M. & Zuker, C. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520, 349–352 (2015). https://doi.org/10.1038/nature14108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14108
This article is cited by
-
Long-term health outcomes associated with hydration status
Nature Reviews Nephrology (2024)
-
TMEM63B channel is the osmosensor required for thirst drive of interoceptive neurons
Cell Discovery (2024)
-
Evaluation of thirst severity, death anxiety, and complementary and supportive therapy use as predictors of urinary incontinence-related quality of life in older adults
European Geriatric Medicine (2023)
-
CPT1A in AgRP neurons is required for sex-dependent regulation of feeding and thirst
Biology of Sex Differences (2023)
-
Exploring the neurobiology of the premonitory phase of migraine preclinically – a role for hypothalamic kappa opioid receptors?
The Journal of Headache and Pain (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.