Abstract
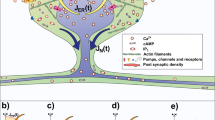
Dendritic spines are the nearly ubiquitous site of excitatory synaptic input onto neurons1,2 and as such are critically positioned to influence diverse aspects of neuronal signalling. Decades of theoretical studies have proposed that spines may function as highly effective and modifiable chemical and electrical compartments that regulate synaptic efficacy, integration and plasticity3,4,5,6,7,8. Experimental studies have confirmed activity-dependent structural dynamics and biochemical compartmentalization by spines9,10,11,12. However, there is a longstanding debate over the influence of spines on the electrical aspects of synaptic transmission and dendritic operation3,4,5,6,7,8,13,14,15,16,17,18. Here we measure the amplitude ratio of spine head to parent dendrite voltage across a range of dendritic compartments and calculate the associated spine neck resistance (Rneck) for spines at apical trunk dendrites in rat hippocampal CA1 pyramidal neurons. We find that Rneck is large enough (∼500 MΩ) to amplify substantially the spine head depolarization associated with a unitary synaptic input by ∼1.5- to ∼45-fold, depending on parent dendritic impedance. A morphologically realistic compartmental model capable of reproducing the observed spatial profile of the amplitude ratio indicates that spines provide a consistently high-impedance input structure throughout the dendritic arborization. Finally, we demonstrate that the amplification produced by spines encourages electrical interaction among coactive inputs through an Rneck-dependent increase in spine head voltage-gated conductance activation. We conclude that the electrical properties of spines promote nonlinear dendritic processing and associated forms of plasticity and storage, thus fundamentally enhancing the computational capabilities of neurons19,20,21.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ramón y Cajal, S. Estructura de los centros nerviosos de las aves. Rev. Trim. Histol. Norm. Patol. 1, 1–10 (1888)
Bourne, J. N. & Harris, K. M. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 31, 47–67 (2008)
Rall, W. Theory of physiological properties of dendrites. Ann. NY Acad. Sci. 96, 1071–1092 (1962)
Rall, W. in Cellular Mechanisms Subserving Changes in Neuronal Activity (ed Woody, C. D. et al.) Brain Information Service Report No. 3 (Univ. of California, 1974)
Miller, J. P., Rall, W. & Rinzel, J. Synaptic amplification by active membrane in dendritic spines. Brain Res. 325, 325–330 (1985)
Koch, C. & Zador, A. The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. J. Neurosci. 13, 413–422 (1993)
Yuste, R. Dendritic spines and distributed circuits. Neuron 71, 772–781 (2011)
Hao, J. & Oertner, T. G. Depolarization gates spine calcium transients and spike-timing-dependent potentiation. Curr. Opin. Neurobiol. 22, 509–515 (2012)
Guthrie, P. B., Segal, M. & Kater, S. B. Independent regulation of calcium revealed by imaging dendritic spines. Nature 354, 76–80 (1991)
Yuste, R. & Denk, W. Dendritic spines as basic functional units of neuronal integration. Nature 375, 682–684 (1995)
Yasuda, R. & Murakoshi, H. The mechanisms underlying the spatial spreading of signaling activity. Curr. Opin. Neurobiol. 21, 313–321 (2011)
Chen, Y. & Sabatini, B. L. Signaling in dendritic spines and spine microdomains. Curr. Opin. Neurobiol. 22, 389–396 (2012)
Svoboda, K., Tank, D. W. & Denk, W. Direct measurement of coupling between dendritic spines and shaft. Science 272, 716–719 (1996)
Grunditz, A., Holbro, N., Tian, L., Zuo, Y. & Oertner, T. G. Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. J. Neurosci. 28, 13457–13466 (2008)
Bloodgood, B. L. & Sabatini, B. L. Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310, 866–869 (2005)
Palmer, L. M. & Stuart, G. J. Membrane potential changes in dendritic spines during action potentials and synaptic input. J. Neurosci. 29, 6897–6903 (2009)
Bloodgood, B. L., Giessel, A. J. & Sabatini, B. L. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biol. 7, e1000190 (2009)
Tsay, D. & Yuste, R. On the electrical function of dendritic spines. Trends Neurosci. 27, 77–83 (2004)
London, M. & Häusser, M. Dendritic computation. Annu. Rev. Neurosci. 28, 503–532 (2005)
Wu, X. E. & Mel, B. W. Capacity-enhancing synaptic learning rules in a medial temporal lobe online learning model. Neuron 62, 31–41 (2009)
Legenstein, R. & Maass, W. Branch-specific plasticity enables self-organization of nonlinear computation in single neurons. J. Neurosci. 31, 10787–10802 (2011)
Magee, J. C. & Cook, E. P. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nature Neurosci. 3, 895–903 (2000)
Bloodgood, B. L. & Sabatini, B. L. Nonlinear regulation of unitary synaptic signals by CaV2. 3 voltage-sensitive calcium channels located in dendritic spines. Neuron 53, 249–260 (2007)
Jaffe, D. B. & Carnevale, N. T. Passive normalization of synaptic integration influenced by dendritic architecture. J. Neurophysiol. 82, 3268–3285 (1999)
Gulledge, A. T., Carnevale, N. T. & Stuart, G. J. Electrical advantages of dendritic spines. PLoS ONE 7, e36007 (2012)
Branco, T., Clark, B. A. & Häusser, M. Dendritic discrimination of temporal input sequences in cortical neurons. Science 329, 1671–1675 (2010)
Losonczy, A., Makara, J. K. & Magee, J. C. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452, 436–441 (2008)
Makara, J. K., Losonczy, A., Wen, Q. & Magee, J. C. Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nature Neurosci. 12, 1485–1487 (2009)
Harvey, C. D. & Svoboda, K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450, 1195–1200 (2007)
Gasparini, S. & Magee, J. C. State-dependent dendritic computation in hippocampal CA1 pyramidal neurons. J. Neurosci. 26, 2088–2100 (2006)
Ji, N., Magee, J. C. & Betzig, E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nature Methods 5, 197–202 (2008)
Johnston, D. & Wu, S. Foundations of Cellular Neurophysiology Ch. 13 400–411 (MIT Press, 1995)
Hines, M. L. & Carnevale, N. T. The neuron simulation environment. Neural Comput. 9, 1179–1209 (1997)
Golding, N. L., Kath, W. L. & Spruston, N. Dichotomy of action-potential backpropagation in CA1 pyramidal neuron dendrites. J. Neurophysiol. 86, 2998–3010 (2001)
Golding, N. L., Mickus, T. J., Katz, Y., Kath, W. L. & Spruston, N. Factors mediating powerful voltage attenuation along CA1 pyramidal neuron dendrites. J. Physiol. (Lond.) 568, 69–82 (2005)
Magee, J. C. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nature Neurosci. 2, 508–514 (1999)
Hoffman, D. A., Magee, J. C., Colbert, C. M. & Johnston, D. K. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875 (1997)
Katz, Y. et al. Synapse distribution suggests a two-stage model of dendritic integration in CA1 pyramidal neurons. Neuron 63, 171–177 (2009)
Dowell, M. & Jarratt, P. A modified regula falsi method for computing the root of an equation. Bit Numerical Mathematics 11, 168–174 (1971)
Acknowledgements
We thank A. Milstein, S. Gale and R. Chitwood for help in creating analysis tools and G. Murphy, S. Williams and D. Johnston for comments on the manuscript. This work was supported by the Howard Hughes Medical Institute, the National Institutes of Health (NS-046064, NS-077601) and the Wellcome Trust (International Senior Research Fellowship to J.K.M., grant number 090915).
Author information
Authors and Affiliations
Contributions
M.T.H., J.K.M. and J.C.M. conceived the project and designed the experiments. M.T.H. and J.K.M. performed all experiments and data analysis. N.S., W.L.K. and J.C.M. performed computer simulations. M.T.H., J.K.M. and J.C.M. wrote the paper with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 and Supplementary Text and Data, which includes a Supplementary Discussion. (PDF 6317 kb)
Rights and permissions
About this article
Cite this article
Harnett, M., Makara, J., Spruston, N. et al. Synaptic amplification by dendritic spines enhances input cooperativity. Nature 491, 599–602 (2012). https://doi.org/10.1038/nature11554
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11554
This article is cited by
-
A GPU-based computational framework that bridges neuron simulation and artificial intelligence
Nature Communications (2023)
-
Excitation–transcription coupling, neuronal gene expression and synaptic plasticity
Nature Reviews Neuroscience (2023)
-
Aβ42 oligomers trigger synaptic loss through CAMKK2-AMPK-dependent effectors coordinating mitochondrial fission and mitophagy
Nature Communications (2022)
-
Two-photon calcium imaging of neuronal activity
Nature Reviews Methods Primers (2022)
-
An astrocytic signaling loop for frequency-dependent control of dendritic integration and spatial learning
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.