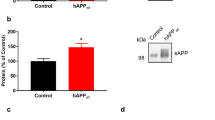
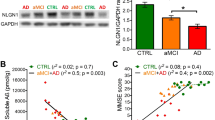
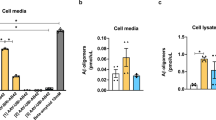
Abstract
Amyloid-β oligomers may cause cognitive deficits in Alzheimer’s disease by impairing neuronal NMDA-type glutamate receptors, whose function is regulated by the receptor tyrosine kinase EphB2. Here we show that amyloid-β oligomers bind to the fibronectin repeats domain of EphB2 and trigger EphB2 degradation in the proteasome. To determine the pathogenic importance of EphB2 depletions in Alzheimer’s disease and related models, we used lentiviral constructs to reduce or increase neuronal expression of EphB2 in memory centres of the mouse brain. In nontransgenic mice, knockdown of EphB2 mediated by short hairpin RNA reduced NMDA receptor currents and impaired long-term potentiation in the dentate gyrus, which are important for memory formation. Increasing EphB2 expression in the dentate gyrus of human amyloid precursor protein transgenic mice reversed deficits in NMDA receptor-dependent long-term potentiation and memory impairments. Thus, depletion of EphB2 is critical in amyloid-β-induced neuronal dysfunction. Increasing EphB2 levels or function could be beneficial in Alzheimer’s disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
16 August 2011
In Supplementary Figure 11, the legend on the y-axis for panels a and b has been corrected to be 'Relative level' instead of 'ng/ml'.
References
Walsh, D. M. & Selkoe, D. J. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44, 181–193 (2004)
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Med. 14, 837–842 (2008)
Kamenetz, F. et al. APP processing and synaptic function. Neuron 37, 925–937 (2003)
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004)
Ikonomovic, M. D. et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer’s disease. Exp. Neurol. 160, 194–204 (1999)
Sze, C., Bi, H., Kleinschmidt-DeMasters, B. K., Filley, C. M. & Martin, L. J. N-Methyl-d-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J. Neurol. Sci. 182, 151–159 (2001)
Palop, J. J. et al. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J. Neurosci. 25, 9686–9693 (2005)
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711 (2007)
Simon, A. M. et al. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. J. Alzheimers Dis. 17, 773–786 (2009)
Henderson, J. T. et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron 32, 1041–1056 (2001)
Dalva, M. B. et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 945–956 (2000)
Takasu, M. A., Dalva, M. B., Zigmond, R. E. & Greenberg, M. E. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295, 491–495 (2002)
Chen, Y., Fu, A. K. & Ip, N. Y. Bidirectional signaling of ErbB and Eph receptors at synapses. Neuron Glia Biol. 4, 211–221 (2008)
Grunwald, I. C. et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 32, 1027–1040 (2001)
Fleischmann, A. et al. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J. Neurosci. 23, 9116–9122 (2003)
Litterst, C. et al. Ligand binding and calcium influx induce distinct ectodomain/γ-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 282, 16155–16163 (2007)
Wakabayashi, K., Honer, W. G. & Masliah, E. Synapse alterations in the hippocampal-entorhinal formation in Alzheimer’s disease with and without Lewy body disease. Brain Res. 667, 24–32 (1994)
Scheff, S. W. & Price, D. A. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J. Alzheimers Dis. 9, 101–115 (2006)
Mueller-Steiner, S. et al. Anti-amyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron 51, 703–714 (2006)
Sun, B. et al. Imbalance between GABAergic and glutamatergic transmissions impairs adult neurogenesis in an animal model of Alzheimer’s disease. Cell Stem Cell 5, 624–633 (2009)
Shemer, I. et al. Non-fibrillar β-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur. J. Neurosci. 23, 2035–2047 (2006)
Ashe, K. H. & Zahs, K. R. Probing the biology of Alzheimer’s disease in mice. Neuron 66, 631–645 (2010)
Colino, A. & Malenka, R. C. Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro . J. Neurophysiol. 69, 1150–1159 (1993)
Harris, J. A. et al. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer’s disease are independent of caspase cleavage of the amyloid precursor protein. J. Neurosci. 30, 372–381 (2010)
Sanchez-Mejia, R. O. et al. Phospholipase A2 reduction ameliorates cognitve deficits in mouse model of Alzheimer’s disease. Nature Neurosci. 11, 1311–1318 (2008)
Meilandt, W. J. et al. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 28, 5007–5017 (2008)
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007)
Nguyen, P. V., Abel, T., Kandel, E. R. & Bourtchouladze, R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn. Mem. 7, 170–179 (2000)
Nakajima, R. et al. Comprehensive behavioral phenotyping of calpastatin-knockout mice. Mol. Brain 1, 7 (2008)
Potter, M. C. et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 35, 1734–1742 (2010)
Terashima, A. et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 57, 872–882 (2008)
Snyder, E. M. et al. Regulation of NMDA receptor trafficking by amyloid-β. Nature Neurosci. 8, 1051–1058 (2005)
Kurup, P. et al. Aβ-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J. Neurosci. 30, 5948–5957 (2010)
Bonifazi, P. et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424 (2009)
Han, J. H. et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009)
Li, C. Y., Poo, M. M. & Dan, Y. Burst spiking of a single cortical neuron modifies global brain state. Science 324, 643–646 (2009)
Rockenstein, E. M. et al. Levels and alternative splicing of amyloid β protein precursor (APP) transcripts in brains of transgenic mice and humans with Alzheimer’s disease. J. Biol. Chem. 270, 28257–28267 (1995)
Mucke, L. et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000)
Koo, E. H. & Squazzo, S. L. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J. Biol. Chem. 269, 17386–17389 (1994)
Walsh, D. M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo . Nature 416, 535–539 (2002)
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates. (Academic, 1997)
Xia, Z., Dudek, H., Miranti, C. K. & Greenberg, M. E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996)
Laurén, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009)
Alfa Cisse, M. et al. M1 and M3 muscarinic receptors control physiological processing of cellular prion by modulating Alzheimer’s disease AM17 phosphorylation and activity. J. Neurosci. 27, 4083–4092 (2007)
Wu, J., Rush, A., Rowan, M. J. & Anwyl, R. NMDA receptor- and metabotropic glutamate receptor-dependent synaptic plasticity induced by high frequency stimulation in the rat dentate gyrus in vitro . J. Physiol. (Lond.) 533, 745–755 (2001)
Raber, J. et al. Hypothalamic-pituitary-adrenal function in Apoe−/− mice: possible role in behavioral and metabolic alterations. J. Neurosci. 20, 2064–2071 (2000)
Raber, J., LeFevour, A., Buttini, M. & Mucke, L. Androgens protect against Apolipoprotein E4-induced cognitive deficits. J. Neurosci. 22, 5204–5209 (2002)
Dere, E., Huston, J. P. & De Souza Silva, M. A. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res. Protoc. 16, 10–19 (2005)
Benice, T., Rizk, A., Kohama, S., Pfankuch, T. & Raber, J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 137, 413–423 (2006)
Johnson-Wood, K. et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl Acad. Sci. USA 94, 1550–1555 (1997)
Acknowledgements
We thank I. Ethell for the plasmid encoding the Flag-tagged EphB2 receptor; D. J. Selkoe and D. Walsh for CHO-7PA2 cells; S. Finkbeiner for the plasmid encoding the NMDA receptor subunit NR1; J. Palop for comments; H. Solanoy, M. Thwin and X. Wang for technical support; G. Howard and S. Ordway for editorial review; J. Carroll for preparation of graphics; and M. Dela Cruz for administrative assistance. The study was supported by NIH grants AG011385, AG022074 and NS041787 to L.M., a fellowship from the McBean Family Foundation to M.C., and the National Center for Research Resources Grant RR18928-01 to the Gladstone Institutes.
Author information
Authors and Affiliations
Contributions
M.C. and L.M. conceptualized the study. M.C., B.H., J.H. and N.D. performed experiments, and all authors participated in designing experiments and in analysing and interpreting data. M.C., B.H. and L.M. wrote the manuscript. L.M. supervised the project.
Corresponding author
Ethics declarations
Competing interests
L.M. serves on the Scientific Advisory Boards of AgeneBio, Inc., Neuropore Therapies, Inc. and Probiodrug A.G.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-15 with legends, Supplementary Tables 1-2, Supplementary Methods and additional references. This file was replaced on 16 August 2011. (PDF 6074 kb)
Rights and permissions
About this article
Cite this article
Cissé, M., Halabisky, B., Harris, J. et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469, 47–52 (2011). https://doi.org/10.1038/nature09635
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09635
This article is cited by
-
The Q/R editing site of AMPA receptor GluA2 subunit acts as an epigenetic switch regulating dendritic spines, neurodegeneration and cognitive deficits in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Synaptic degeneration in Alzheimer disease
Nature Reviews Neurology (2023)
-
Function and therapeutic value of astrocytes in neurological diseases
Nature Reviews Drug Discovery (2022)
-
A joint analysis using exome and transcriptome data identifies candidate polymorphisms and genes involved with umbilical hernia in pigs
BMC Genomics (2021)
-
Is kallikrein-8 a blood biomarker for detecting amnestic mild cognitive impairment? Results of the population-based Heinz Nixdorf Recall study
Alzheimer's Research & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.