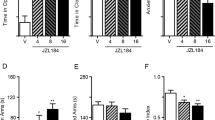
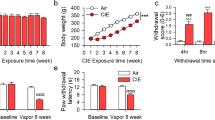
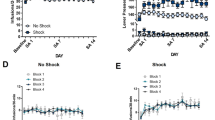
Abstract
Acute stress suppresses pain by activating brain pathways that engage opioid or non-opioid mechanisms. Here we show that an opioid-independent form of this phenomenon, termed stress-induced analgesia1, is mediated by the release of endogenous marijuana-like (cannabinoid) compounds in the brain. Blockade of cannabinoid CB1 receptors in the periaqueductal grey matter of the midbrain prevents non-opioid stress-induced analgesia. In this region, stress elicits the rapid formation of two endogenous cannabinoids, the lipids 2-arachidonoylglycerol2 (2-AG) and anandamide3. A newly developed inhibitor of the 2-AG-deactivating enzyme, monoacylglycerol lipase4,5, selectively increases 2-AG concentrations and, when injected into the periaqueductal grey matter, enhances stress-induced analgesia in a CB1-dependent manner. Inhibitors of the anandamide-deactivating enzyme fatty-acid amide hydrolase6, which selectively elevate anandamide concentrations, exert similar effects. Our results indicate that the coordinated release of 2-AG and anandamide in the periaqueductal grey matter might mediate opioid-independent stress-induced analgesia. These studies also identify monoacylglycerol lipase as a previously unrecognized therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewis, J. W., Cannon, J. T. & Liebeskind, J. C. Opioid and nonopioid mechanisms of stress analgesia. Science 208, 623–625 (1980)
Mechoulam, R. et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 (1995)
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992)
Dinh, T. P. et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA 99, 10819–10824 (2002)
Dinh, T. P., Kathuria, S. & Piomelli, D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol. Pharmacol. 66, 1260–1264 (2004)
Cravatt, B. F. et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 (1996)
Walker, J. M. & Hohmann, A. G. in Cannabinoids—Handbook of Experimental Pharmacology (ed. Pertwee, R.) 509–554 (Springer, Berlin, 2005)
Akil, H., Young, E., Walker, J. M. & Watson, S. J. The many possible roles of opioids and related peptides in stress-induced analgesia. Ann. NY Acad. Sci. 467, 140–153 (1986)
Herkenham, M. et al. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563–583 (1991)
Zimmer, A., Zimmer, A. M., Hohmann, A. G., Herkenham, M. & Bonner, T. I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl Acad. Sci. USA 96, 5780–5785 (1999)
Hohmann, A. G., Martin, W. J., Tsou, K. & Walker, J. M. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212–2. Life Sci. 56, 2111–2118 (1995)
Hohmann, A. G., Tsou, K. & Walker, J. M. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J. Neurophysiol. 81, 575–583 (1999)
Martin, W. J., Hohmann, A. G. & Walker, J. M. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J. Neurosci. 16, 6601–6611 (1996)
Meng, I. D., Manning, B. H., Martin, W. J. & Fields, H. L. An analgesia circuit activated by cannabinoids. Nature 395, 381–383 (1998)
Calignano, A., La Rana, G., Giuffrida, A. & Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 394, 277–281 (1998)
Terman, G. W., Lewis, J. W. & Liebeskind, J. C. Two opioid forms of stress analgesia: studies of tolerance and cross-tolerance. Brain Res. 368, 101–106 (1986)
Walker, J. M., Huang, S. M., Strangman, N. M., Tsou, K. & Sañudo-Peña, M. C. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl Acad. Sci. USA 96, 12198–12203 (1999)
Martin, W. J., Patrick, S. L., Coffin, P. O., Tsou, K. & Walker, J. M. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 56, 2103–2109 (1995)
Cannon, J. T., Prieto, G. J., Lee, A. & Liebeskind, J. C. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 243, 315–321 (1982)
Tsou, K., Brown, S., Sañudo-Peña, M. C., Mackie, K. & Walker, J. M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 (1998)
Kathuria, S. et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nature Med. 1, 76–81 (2003)
Mor, M. et al. Cyclohexylcarbamic acid 3′- or 4′-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure–activity relationships, and molecular modeling studies. J. Med. Chem. 47, 4998–5008 (2004)
Stella, N., Schweitzer, P. & Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388, 773–778 (1997)
Kozak, K. R., Prusakiewicz, J. J. & Marnett, L. J. Oxidative metabolism of endocannabinoids by COX-2. Curr. Pharm. Des. 10, 659–667 (2004)
De Petrocellis, L., Bisogno, T., Davis, J. B., Pertwee, R. G. & Di Marzo, V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 483, 52–56 (2000)
Vaughan, C. W., Connor, M., Bagley, E. E. & Christie, M. J. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol. Pharmacol. 57, 288–295 (2000)
Valverde, O., Ledent, C., Beslot, F., Parmentier, M. & Roques, B. P. Reduction of stress-induced analgesia but not of exogenous opioid effects in mice lacking CB1 receptors. Eur. J. Neurosci. 12, 533–539 (2000)
Piomelli, D. The molecular logic of endocannabinoid signalling. Nature Rev. Neurosci. 4, 873–884 (2003)
Tarzia, G. et al. Design, synthesis, and structure–activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J. Med. Chem. 46, 2352–2360 (2003)
Giuffrida, A., Rodríguez de Fonseca, F. & Piomelli, D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal. Biochem. 280, 87–93 (2000)
Acknowledgements
The assistance of the Centro di Calcolo at the University of Parma is gratefully acknowledged. This research was supported by grants from the National Institute on Drug Abuse (A.G.H., D.P.) and from the MIUR and the Universities of Parma and Urbino ‘Carlo Bo’.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
A patent has been filed on their behalf by the University of California at Irvine.
Supplementary information
Supplementary Figure S1
Systemic administration of the CB1 antagonist AM251 suppresses non-opioid stress-induced antinociception. (PDF 9 kb)
Supplementary Figure S2
Rimonabant does not alter tail-flick latencies in the absence of the stressor. (PDF 8 kb)
Supplementary Figure S3
Non-opioid stress-induced antinociception is unaffected by morphine tolerance. (PDF 7 kb)
Supplementary Figure S4
Rimonabant does not alter stress-induced antinociception following microinjection in the lateral/ventrolateral PAG. (PDF 29 kb)
Supplementary Figure S5
Administration of rimonabant into the lateral ventricle does not affect opioid-independent stress-induced antinociception. (PDF 10 kb)
Supplementary Figure S6
Representative LC/MS tracings for selected ions characteristic of endogenous 2-AG, synthetic [2H8]-2-AG standard, endogenous anandamide and synthetic [2H4]-2-anandamide standard. (PDF 33 kb)
Supplementary Figure S7
Coronal reconstructions of injection sites following microinjection of URB602 in the (a) dorsolateral or (b,c) lateral/ventrolateral PAG. (PDF 80 kb)
Supplementary Figure S8
Coronal reconstructions of injection sites following microinjection of URB597 in the dorsolateral PAG. (PDF 41 kb)
Supplementary Figure S9
Inhibition of MGL enhances nonopioid stress-induced analgesia. (PDF 54 kb)
Rights and permissions
About this article
Cite this article
Hohmann, A., Suplita, R., Bolton, N. et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 435, 1108–1112 (2005). https://doi.org/10.1038/nature03658
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03658
This article is cited by
-
Cannabinoids as a Potential Alternative to Opioids in the Management of Various Pain Subtypes: Benefits, Limitations, and Risks
Pain and Therapy (2023)
-
A monoacylglycerol lipase inhibitor showing therapeutic efficacy in mice without central side effects or dependence
Nature Communications (2023)
-
Acquisition of threat responses are associated with elevated plasma concentration of endocannabinoids in male humans
Neuropsychopharmacology (2022)
-
Neuronal Nitric Oxide Synthase Critically Regulates the Endocannabinoid Pathway in the Murine Cerebellum During Development
The Cerebellum (2022)
-
Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons
Nature Neuroscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.