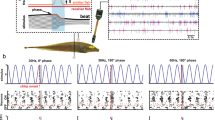
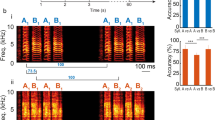
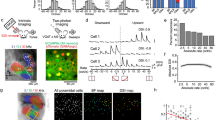
Abstract
Animals have developed stereotyped communication calls to which specific sensory neurons are well tuned1,2. These communication calls must be discriminated from environmental signals such as those produced by prey. Sensory systems might have evolved neural circuitry to encode both categories. In weakly electric fish, prey and communication signals differ in their spatial extent and frequency content3,4. Here we show that stimuli of different spatial extents mimicking prey and communication signals cause a switch in the frequency tuning and spike-timing precision of electrosensory pyramidal neurons, resulting in the selective and optimal encoding of both stimulus categories. As in other sensory systems5, pyramidal neurons respond only to stimuli located within a restricted region of space known as the classical receptive field (CRF)6. In some systems, stimulation outside the CRF but within a non-classical receptive field (nCRF) can modulate the neural response to CRF stimulation even though nCRF stimulation alone fails to elicit responses7,8. We show that pyramidal neurons possess a nCRF and that it can modulate the response to CRF stimuli to induce this neurobiological switch in frequency tuning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rieke, F., Bodnar, D. A. & Bialek, W. Naturalistic stimuli increase the rate and efficiency of information transmission by primary auditory afferents. Proc. R. Soc. Lond. B 262, 259–265 (1995)
Machens, C. K. et al. Representation of acoustic communication signals by insect auditory neurons. J. Neurosci. 21, 3215–3227 (2001)
Nelson, M. E. & MacIver, M. A. Prey capture in the weakly electric fish Apteronotus leptorhynchus: sensory acquisition strategies and electrosensory consequences. J. Exp. Biol. 202, 1195–1203 (1999)
Zupanc, G. K. H. & Maler, L. Evoked chirping in the weakly electric fish Apteronotus leptorhynchus: a quantitative biophysical analysis. Can. J. Zool. 71, 2301–2310 (1993)
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962)
Bastian, J., Chacron, M. J. & Maler, L. Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity. J. Neurosci. 22, 4577–4590 (2002)
Sillito, A. M., Grieve, K. L., Jones, H. E., Cudeiro, J. & Davis, J. Visual cortical mechanisms detecting focal orientation discontinuities. Nature 378, 492–496 (1995)
Vinje, W. & Gallant, J. L. Sparse Coding and decorrelation in primary visual cortex during natural vision. Science 287, 1273–1276 (2000)
Simoncelli, E. P. & Olshausen, B. A. Natural image statistics and neural representation. Annu. Rev. Neurosci. 24, 1193–1216 (2001)
Voss, R. F. & Clarke, J. ‘1/f noise’ in music: music from 1/f noise. J. Acoust. Soc. Am. 63, 258–263 (1978)
Bastian, J. Electrolocation. I. How the electroreceptors of Apteronotus albifrons code for moving objects and other electrical stimuli. J. Comp. Physiol. A 144, 465–479 (1981)
Gabbiani, F., Metzner, W., Wessel, R. & Koch, C. From stimulus encoding to feature extraction in weakly electric fish. Nature 384, 564–567 (1996)
Maler, L., Sas, E. K. & Rogers, J. The cytology of the posterior lateral line lobe of high frequency weakly electric fish (Gymnotoidei): Dendritic differentiation and synaptic specificity in a simple cortex. J. Comp. Neurol. 195, 87–139 (1981)
Rieke, F., Warland, D., de Ruyter van Steveninck, R. R. & Bialek, W. Spikes: Exploring the Neural Code (MIT, Cambridge, Massachusetts, 1996)
Borst, A. & Theunissen, F. Information theory and neural coding. Nature Neurosci. 2, 947–957 (1999)
Mainen, Z. F. & Sejnowski, T. J. Reliability of spike timing in neocortical neurons. Science 268, 1503–1506 (1995)
de Ruyter van Steveninck, R. R., Lewen, G. D., Strong, S. P., Koberle, R. & Bialek, W. Reproducibility and variability in neural spike trains. Science 275, 1805–1808 (1997)
Maler, L. & Mugnaini, E. Correlating gamma-aminobutyric acidergic circuits and sensory function in the electrosensory lateral line lobe of a gymnotiform fish. J. Comp. Neurol. 345, 224–252 (1994)
Berman, N. J. & Maler, L. Neural architecture of the electrosensory lateral line lobe: Adaptations for coincidence detection, a sensory searchlight and frequency-dependent adaptive filtering. J. Exp. Biol. 202, 1243–1253 (1999)
Crampton, W. G. R. Electric signal design and habitat preferences in a species rich assembly of gymnotiform fishes from the upper Amazon basin. Anais Acad. Bras. Cienc. 70, 805–847 (1998)
Zhang, H., Xu, J. & Feng, A. S. Effects of GABA-mediated inhibition on direction-dependent frequency tuning in the frog inferior colliculus. J. Comp. Physiol. 184, 85–98 (1999)
Macleod, K. & Laurent, G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assembies. Science 274, 976–979 (1996)
Doiron, B., Chacron, M. J., Maler, L., Longtin, A. & Bastian, J. Inhibitory feedback required for network burst responses to communication but not prey stimuli. Nature 421, 539–543 (2003)
Carr, C. E., Maler, L. & Sas, E. Peripheral organization and central projections of the electrosensory organs in gymnotiform fish. J. Comp. Neurol. 211, 139–153 (1982)
Heiligenberg, W. & Dye, J. Labelling of electrosensory afferents in a gymnotid fish by intracellular injection of HRP: The mystery of multiple maps. J. Comp. Physiol. A 148, 287–296 (1982)
Metzner, W. & Heiligenberg, W. The coding of signals in the electric communication of the gymnotiform fish Eigenmannia: From electroreceptors to neurons in the torus semicircularis of the midbrain. J. Comp. Physiol. A 169, 135–150 (1991)
Metzner, W. & Juranek, J. A sensory brain map for each behavior? Proc. Natl Acad. Sci. USA 26, 14798–14803 (1997)
Heiligenberg, W. Neural Nets in Electric Fish (MIT, Cambridge, Massachusetts, 1991)
Bastchelet, E. Circular Statistics in Biology (Academic, New York, 1981)
Gabbiani, F. Coding of time varying signals in spike trains of linear and half-wave rectifying neurons. Network Comput. Neural Sys. 7, 61–85 (1996)
Acknowledgements
We thank A.-M. Oswald, J. Lewis and B. Lindner for their reading the manuscript. This research was supported by NSERC (M.J.C., B.D., A.L.), CIHR (L.M., A.L.) and NIH (J.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Chacron, M., Doiron, B., Maler, L. et al. Non-classical receptive field mediates switch in a sensory neuron's frequency tuning. Nature 423, 77–81 (2003). https://doi.org/10.1038/nature01590
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01590
This article is cited by
-
Visual surround suppression at the neural and perceptual levels
Cognitive Neurodynamics (2023)
-
Morphology and receptive field organization of a temporal processing region in Apteronotus albifrons
Journal of Comparative Physiology A (2022)
-
Synergistic population coding of natural communication stimuli by hindbrain electrosensory neurons
Scientific Reports (2021)
-
Information filtering by coincidence detection of synchronous population output: analytical approaches to the coherence function of a two-stage neural system
Biological Cybernetics (2020)
-
High-resolution behavioral mapping of electric fishes in Amazonian habitats
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.