Abstract
Background:
FKBP51 is overexpressed in melanoma and impacts tumour cell properties. However, its comprehensive role in melanoma pathogenesis and underlying mechanism(s) remain elusive.
Methods:
FKBP51 was stably silenced in aggressive melanoma cell lines and its effect examined in vitro and in mouse model. Histological/immunohistochemical analyses were performed to confirm metastasis, angiogenesis and neutrophil infiltration. Gene expression was analyzed by qRT–PCR, immunoblot and/or ELISA. NF-κB transcriptional activity and promoter binding were monitored by luciferase-based promoter-reporter and ChIP assays, respectively. Interleukin (IL)-8 inhibition was achieved by gene silencing or neutralising-antibody treatment.
Results:
FKBP51 silencing reduced melanoma growth, metastasis, angiogenesis and neutrophil infiltration and led to IL-8 downregulation through NF-κB suppression in cell lines and tumour xenografts. IL-8 inhibition drastically decreased growth, migration and invasiveness of FKPB51-overexpressing cells; whereas its treatment partially restored the suppressed phenotypes of FKBP51-silenced melanoma cells. Interleukin-8 depletion in conditioned medium (CM) of FKBP51-overexpressing melanoma cells inhibited endothelial cell proliferation and capillary-like structure formation, whereas its treatment promoted these effects in endothelial cells cultured in CM of FKBP51-silenced melanoma cells.
Conclusions:
FKBP51 promotes melanoma growth, metastasis and angiogenesis, and IL-8 plays a key role in these processes. Thus, targeting of FKBP51 or its upstream or downstream regulatory pathways could lead to effective therapeutic strategies against melanoma.
Similar content being viewed by others
Main
Melanoma is an aggressive form of skin cancer in human. It is the sixth most commonly diagnosed cancer, and one of the leading causes of skin cancer-related deaths in human in the United States (Sun et al, 2015). Incidence and mortality rates of melanoma have increased tremendously during the past several decades. According to an estimate, nearly 73 870 new cases of melanoma will be diagnosed and approximately 9940 patients will die from this malignancy in 2015 (Siegel et al, 2015). Moreover, the 5-year survival rate of melanoma patients with distant metastasis is 15% with the median survival of 6–9 months (Rodic et al, 2014). Together, these facts clearly highlight the need to develop effective preventive and/or therapeutic strategies against this deadly malignancy. To be able to make progress in these areas, we need to first identify molecular players involved in melanoma initiation and progression as well as characterise the mechanisms through which they confer their impact on melanoma phenotype. So far, several molecular aberrations have been recorded in malignant melanoma and have been characterised for their involvement in pathogenesis.
FK506-binding protein 51 (FKBP51, also referred as FKBP5) belongs to a highly conserved family of immunophilins having cis-trans peptidyl-prolyl isomerase activity (Galat, 1993; Jiang et al, 2008). In recent years, FKBP51 has emerged as a chief regulator of immunity, hormonal physiology, drug sensitivity and biological processes involved in cell survival, protein synthesis, folding as well as in trafficking (Storer et al, 2011). Structurally, FKBP51 contains multiple functional domains (Storer et al, 2011). The N-terminal FK1 domain possesses the PPIase function, which is inhibited upon direct binding of FK506 (Bracher et al, 2013). The FK2 is PPIase-like domain and does not have much PPIase activity and is not inhibited by FK506 (Storer et al, 2011). The C-terminus of FKBP51 contains a three-unit domain of tetratricopeptide repeats that mediates its interactions with other proteins (Sinars et al, 2003). Recent studies have shown that FKBP51 is overexpressed in human melanoma tissues and associated with cell survival and chemoresistance (Romano et al, 2010, 2011). FKBP51 also plays an important role in maintenance of melanoma stemness and metastatic potential (Romano et al, 2013). Despite these advances, the mechanism(s) through which FKBP51 promotes melanoma progression and metastasis has remained largely unknown.
In this study, we investigated the role of FKBP51 in melanoma growth, metastasis and angiogenesis and identified interleukin 8 (IL-8), a chemokine, as a key mediator of its pathological functions. Interleukin-8, also referred as CXCL8, which is not normally produced by melanocytes, exhibits significant overexpression in melanoma along with its cognate receptors, CXCR1 and CXCR2 (Singh et al, 2010). Furthermore, several lines of evidence indicate its multifaceted action in the progression of human malignant melanoma (Wang et al, 1990; Varney et al, 2006; Singh et al, 2009a; Wu et al, 2012). Activation of IL-8 downstream signalling leads to altered expression of several genes involved in cell proliferation, apoptosis resistance and cytoskeletal dynamics (Waugh and Wilson, 2008; Wu et al, 2012). A positive correlation of IL-8 and its cognate receptors expression with melanoma aggressiveness has been demonstrated by us and others (Varney et al, 2006; Gabellini et al, 2009; Singh et al, 2011; Wu et al, 2012) and its inhibition leads to suppressive effects in vitro and in the preclinical studies of melanoma (Huang et al, 2002; Gabellini et al, 2009; Singh et al, 2011). Therefore, identification of IL-8 as a novel downstream target of FKBP51-mediated signalling is significant considering its reported aberrant expression and pathological involvement in melanoma pathogenesis.
Materials and methods
Reagents, plasmid constructs and antibodies
Details of all the reagents, plasmid constructs, siRNAs and antibodies used in this study are provided in Supplementary Materials.
Cell lines and culture conditions
Human melanoma cell lines, A375P and A375SM were obtained from Dr Isaiah J Fidler (University of Texas MD Anderson Cancer Center, Houston, TX, USA); FEMX-V; FEMX-1 and-FEMX-DR were generously gifted by Dr Øystein Fodstad (Oslo University Hospital, Oslo, Norway), and WM-115, WM266.4, SK-MEL-28, MEWO and human umbilical vein endothelial cells (HUVEC) were from ATCC (Manassas, VA, USA). Cell lines were maintained in DMEM medium supplemented with FBS (10%), penicillin (100 units per ml) and streptomycin (100 μg ml−1) at 37 °C in a humidified atmosphere of 5% CO2. Cells were routinely monitored for their typical morphology, and intermittently tested for mycoplasma.
Transfection
A375SM and-FEMX-1 cells were transfected with plasmids expressing shRNA against FKBP51 (pGFP-V-RS-shFKBP51) and non-targeting-scramble-sequence expressing control vector (pGFP-V-RS-NT) using FuGENE reagent. Stable pooled population were selected in puromycin (2 μg ml−1) containing culture medium. To dissect the role of NF-κB, cells were transiently transfected with constitutively active mutant plasmids of IκB-α and-IKKβ-SSEE along with respective control vectors using FuGENE reagent. For transient silencing, cells were transfected with 50 nM of human IL-8 specific or non-target-scrambled siRNAs.
RNA isolation and reverse transcription polymerase chain reaction (RT–PCR)
Total RNA was extracted using TRIzol reagent and RT–PCR analysis was performed as previously described (Tyagi et al, 2014). Specific sets of primer pairs were used (Supplementary Table S1).
Western blot analysis
Cells were processed for protein extraction and western blotting as described earlier (Tyagi et al, 2014). Specific primary antibodies at different dilutions (1 : 50 to 1 : 1000) followed with HRP-labelled secondary antibodies (1:2000) were used. β-actin (1 : 20 000) probing was used as a loading control.
Cell motility and invasion assay
For motility (1 × 106 cells per well) and invasion (1 × 105 cells per well) assays, cells were plated on uncoated membrane (for motility assay) and on Matrigel-coated membrane (for invasion assay) chambers in serum-deprived culture medium, medium supplemented with FBS (10%) or rIL-8 (10 ng ml−1). Following 16 h incubation, migrated or invaded cells were fixed, stained and observed under microscope and counted in ten random fields of view ( × 200).
In vitro cell growth assay
Cells (1 × 104 cells per well) were seeded and growth was monitored after-96 h using-WST-1 assay kit (Roche Diagnostics, Mannheim, Germany) as described earlier (Bhardwaj et al, 2014). Separately, cells were treated with control-IgG (200 ng ml−1), IL-8-neutralising antibody (200 ng ml−1) or rIL-8 (10 ng ml−1) for 48 h. Following treatment, cells were replenished with fresh treatment media for another 48 h and growth was monitored. For HUVECs proliferation assay, cells (2 × 103 cells per well) were incubated with conditioned medium (CM) obtained from different melanoma cells (NT, FKBP51/IL-8-silenced and IL-8-neutralising antibody (200 ng ml−1) treated cells for 48 h). In addition, HUVECs cells were treated with or without-rIL-8 (10 ng ml−1) in serum-free media for 48 h, and growth was monitored.
Plating efficiency assay
Controls and FKBP51/IL-8-silenced cells (1 × 103 cells per well) were seeded in 6-well plates in complete media. Additionally, control cells were treated with control-IgG (200 ng ml−1) or with-IL-8-neutralising antibody, and FKBP51-silenced cells were treated with rIL-8 (10 ng ml−1). Every third day, media was replaced with fresh culture/treatment media. Following 2 weeks of culturing, colonies were fixed (methanol), stained (crystal violet), photographed and counted using Image analysis software (Gene Tools, Syngene, Frederick, MD, USA).
In vitro angiogenesis assay
Human umbilical vein endothelial cells (1 × 104) were seeded on matrigel-coated plate in CM obtained from controls, shFKBP51/IL-8-silenced and IL-8-neutralising antibody (200 ng ml−1)/control IgG (200 ng ml−1)-treated cells. After 16 h of incubation, capillary-like structure (CLS) formation was observed and counted in ten random fields ( × 100).
Enzyme-linked immunosorbent assay (ELISA)
Cells (1 × 106 per well) were seeded in 6-well plates for 24 h and media replaced with serum-free media. At different intervals (24, 48 and 72 h) culture supernatants were collected and IL-8 levels were determined using human IL-8 ELISA kit.
NF-κB transcriptional activity assay
Transcriptional activity of NF-κB in controls and FKBP51-silenced cells were examined as previously described (Singh et al, 2012).
Nuclear and cytoplasmic fractionation
The cytoplasmic and nuclear extracts were fractioned using the nuclear extract kit as described previously (Singh et al, 2012).
Chromatin immunoprecipitation assay
Binding of NF-κB/p65 to the IL-8 promoter was analyzed by chromatin immunoprecipitation assay using a ChIP-IT enzymatic kit as previously described. Polymerase chain reaction was performed using specific primers sets (Supplementary Table S1), products resolved on a 2.0% agarose gel, and visualised using ethidium-bromide staining.
In vivo tumour growth and experimental lung metastasis
Animal studies were performed after the approval of University of South Alabama Institutional Animal Care and Use Committee (IACUC). Athymic nude mice (Nude-Foxn1nu, stock number 069; 4–6 weeks old) were purchased from Harlan Laboratories (Prattville, AL, USA) and maintained in pathogen-free conditions. A375SM-NT or A375SM-shFKBP51 cells (1 × 106 cells per 0.1 ml of HBSS; n=5) were subcutaneously injected on the right rear flank of mice. Tumour growth was monitored twice a week and tumour volume was calculated using the formula: π/6 × (smaller diameter)2 × (larger diameter). At the end point (45 days post implantation), mice were killed, tumours harvested, weighed, fixed and processed for immunohistochemical analysis. For experimental lung metastasis, A375SM-NT and A375SM-shFKBP51 cells (1 × 106 cells per 0.1 ml of HBSS; n=10) were injected intravenously through tail vein injection. Mice were killed 8 weeks following tumour cell injection and lungs harvested and fixed in Bouin’s solution.
Immunohistochemical and histological analyses
Tumour sections were deparaffinised using EZ-Dewax and rehydrated and processed for staining as described earlier (Tyagi et al, 2014). Mouse anti-human FKBP51 (1 : 200), mouse anti-human IL-8 (1 : 200), rat anti-mouse GR-1 (1 : 50) and biotinylated lectin GS-IB4 (1 : 50) were used for 60 min at room temperature. Subsequently, sections were incubated at room temperature with respective polymer and probe pairs as per manufacturer’s instructions except for GS-IB4. Immunoreactivity was visualised using DAB substrate. Negative control tissues were incubated with all the reagents except primary antibody. The stained cells were visualised under microscope, counted in 10 random fields ( × 200) and photographed. Histological examination followed by haematoxylin and eosin staining was performed on lung tissue sections to examine the presence of metastatic tumour nodules. Tumour nests were visualised under microscope ( × 200) and photographed.
Statistical analysis
All experiments were performed at least three times and numerical data expressed as mean±s.e.m. Wherever appropriate, the data were also subjected to unpaired two-tailed Student’s t test and P<0.05 was considered statistically significant.
Results
FKBP51-induced growth and metastasis of melanoma cells is associated with enhanced angiogenesis and neutrophil infiltration
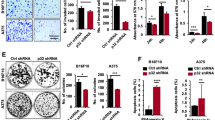
FKBP51 is shown to impact melanoma pathogenesis by promoting tumour cell survival, stemness and metastatic potential (Romano et al, 2010, 2013, 2014). Here, we set out to further explore the functional importance of FKBP51 in melanoma growth, metastasis, angiogenesis and immune cell infiltration. A375SM-shFKBP51 and A375SM-NT cells were subcutaneously implanted into the mice or delivered through tail vein injection. A375SM-shFKBP51 cells implanted subcutaneously exhibited slower tumour growth compared with A375SM-NT cells (Figure 1A). Average volume and weight of tumours developed in A375SM-NT group at the end point were 677.24 mm3 and 1.1 g, respectively, as compared with 78.27 mm3 and 0.14 g in A375SM-shFKBP51 group (Figure 1A and B). Furthermore, in experimental lung metastasis assay, we observed the presence of multiple metastatic tumour nodules (∼10 per mice) in the lungs of all the mice from the control group, whereas no tumour cell nodule was seen in the FKBP51-knockdown group (Figure 1C and D). To further examine the effect of FKBP51 on tumour angiogenesis and neutrophil infiltration, we conducted histochemical analyses. We observed ∼5.5-fold decrease in the number of blood vessels in tumours developed from A375SM-shFKBP51 cells as compared with A375SM-NT cells (Figure 1E). Similarly, neutrophil infiltration was also significantly reduced (∼43.0-fold) in tumours from A375SM-shFKBP51 as compared with the control group (Figure 1F). Together, these data reveal that FKBP51 promotes melanoma growth and metastasis, which is associated with increased angiogenesis and neutrophil infiltration.
Knockdown of FKBP51 inhibits tumour growth, metastasis, angiogenesis and neutrophil infiltration. A375SM-shFKBP51 and A375SM-NT cells were subcutaneously injected on the right rear flank in athymic nude mice (n=5/group) and killed after 45 days. (A) Volume and (B) weight of the tumours from control and FKBP51-silenced group were calculated. (C) For experimental lung metastasis, A375SM-shFKBP51 and A375SM-NT cells were injected intravenously into the nude mice. Following 8 weeks of tumour cell injection, lungs harvested and fixed, and number of metastatic colonies per mouse were counted and plotted. Dark line indicates the median value of the group. (D) Haematoxylin and eosin-stained lung sections showing dense tumour nest in control group whereas FKBP51-silenced group had no gross evidence of deposits of tumour cells. (E and F) In immunostained tumour sections (n=5), number of microvessels and neutrophils were counted in 10 random view fields (magnification × 200). All the data are representative of at least triplicate independent experiments and the data are presented as the mean±s.e.m., *P<0.05.
Expression of FKBP51 positively correlates with IL-8 levels
Previously, we and others have shown that IL-8, a human C-X-C chemokine, plays important roles in melanoma growth, metastasis, vascularisation and recruitment of neutrophils at the tumour site (Huang et al, 2002; Varney et al, 2006; Gabellini et al, 2009; Singh et al, 2009a, 2009b; Wu et al, 2012). Therefore, we examined whether FKBP51 expression impacted IL-8 production by performing immunohistochemical analysis. In tumour tissues, we observed a positive association of FKBP51 and IL-8 expression (Figure 2A). To further confirm this, we silenced FKBP51 expression in another aggressive melanoma cell line (FEMX-1) and examined changes in IL-8 expression by RT–PCR and immunoblot analyses. We also observed decreased mRNA and protein expression of IL-8 in A375SM-shFKBP51 and FEMX-1-shFKBP51 cells relative to their controls (Figure 2B). The amount of IL-8 secreted by the FKBP51-expressing and -silenced cells was also determined by ELISA. Data demonstrate the low levels of IL-8 in the culture supernatant of A375SM-shFKBP51 (∼7.2-, 11.1- and 11.6-folds at 24, 48 and 72 h, respectively) and in FEMX-1-shFKBP51 (∼6.1-, 9.8-, and 10.2-folds at 24, 48 and 72 h, respectively) as compared with their respective control cells (Figure 2C). Interleukin-8 expression was also examined in a panel of melanoma cell lines exhibiting variable expression of FKBP51. The data demonstrate a positive correlation between FKBP51 and IL-8 expression in melanoma cells at both mRNA and protein levels (Supplementary Figure S1). Together, these data indicate a direct association of FKBP51 and IL-8 expression, and suggest that IL-8 may serve as a mediator to regulate FKBP51-promoted melanoma pathogenesis.
Expression of FKBP51 positively correlates with IL-8 levels. (A) Immunohistological analysis of paraffin-embedded melanoma tumours (n=5) for FKBP51 expression (upper panel) and IL-8 expression (lower panel). The data are representative of at least triplicate independent experiments. (B) Expression of FKBP51 and IL-8 at transcript (upper panel) and protein (lower panel) levels was examined by PCR and immunoblot assays, respectively. GAPDH (for PCR) and β-actin (for immunoblot assay) served as internal controls. (C) Cell culture supernatants was collected after 24, 48 and 72 h and processed for ELISA. Data are presented as mean±s.e.m., *P<0.05.
FKBP51 induces IL-8 expression in melanoma cells through activation of NF-κB pathway
To further confirm the association of FKBP51 and IL-8, we investigated the mechanism underlying FKBP51-mediated regulation of IL-8. As IL-8 expression was decreased at the transcript level in FKBP51-silenced cells, we performed in silico analysis of ∼1 kb DNA region 5’ upstream of their coding DNA sequence (GenBank accession number NG029889) using web-based application (ALGGEN-PROMO). We observed a putative binding site (-497 to -507) for NF-κB, a constitutively active transcription factor in melanoma, which is also shown to be regulated by FKBP51 (Romano et al, 2004, 2011). As expected, we observed a decreased transcriptional activity of NF-κB in A375SM-shFKBP51 and FEMX-1-shFKBP51 cells (∼3.8- and 4.3-folds, respectively) as compared with their respective controls (Figure 3A). We also observed decreased nuclear accumulation with a concomitant increase in its cytoplasmic levels in FKBP51-silenced cells associated with decreased phosphorylation and enhanced level of IκB-α (inhibitor of NF-κB) (Figure 3B). Furthermore, we examined the direct binding of NF-κB to IL-8 promoter region in chromatin immunoprecipitation assay, which was decreased in A375SM-shFKBP51 and FEMX-1-shFKBP51 cells (Figure 3C). To further confirm the involvement of NF-κB in FKBP51-mediated regulation of IL-8, we transiently transfected FKBP51-expressing cells with degradation-resistant IκB-α-mutant. In parallel studies, FKBP51-silenced cells were transfected with a constitutively active mutant of IKKβ (IKKβ-SSEE), an upstream kinase of IκB-α. The data reveal that transcriptional activity of NF-κB is suppressed in FKBP51-expressing cells upon transfection of IκB-α-mutant, while the suppressive effect of FKBP51 silencing on the transcriptional activity of NF-κB is abolished following transfection of A375SM-shFKBP51 and FEMX-1-shFKBP51 cells with IKKβ mutant (Supplementary Figure S2). This is accompanied by decreased (in IκB-α MUT-transfected A375SM- and FEMX-1-NT cells) and enhanced (in IKKβ mutant-transfected A375SM- and FEMX-1 -shFKBP51 cells) nuclear accumulation of NF-κB (Figure 3D upper panel). Importantly, decreased expression of IL-8 in IκB-α MUT-transfected FKBP51-expressing cells was observed, whereas expression of IL-8 is restored in A375SM- and FEMX-1-shFKBP51 cells transfected with IKKβ mutant (Figure 3D lower panel). Altogether, these findings confirm that FKBP51 regulates IL-8 through the activation of NF-κB.
FKBP51-activated NF- κ B regulates IL-8 in melanoma cells. (A) Cells were transiently co-transfected with NF-κB-responsive or control reporter plasmids. After 24 h of transfection, firefly and renilla luciferase activities were examined in the FKBP51-silenced or control cells as a measure of NF-κB transcriptional activity and transfection efficiency, respectively. (B) Total, nuclear and cytoplasmic extracts were prepared from FKBP51-silenced or control cells and expression of NF-κB-p65, IκB-α were determined by immunoblot analysis. Laminin, α-tubulin and β-actin were used as a loading control for nuclear, cytoplasmic and total proteins, respectively. (C) Chromatin immunoprecipitation assay was performed as described in materials and methods to examine the binding of NF-κB to IL-8 promoter region. (D) Control cells were transiently transfected with degradation-resistant IκB-α-mutant plasmid and FKBP51-silenced cells were transfected with constitutively active IKKβ-SSEE plasmid. Thereafter, nuclear and total protein lysates were prepared after 24 h and/or 48 h of transfection, and expression levels of NF-κB (in nuclear lysate after 24 h) and IL-8 (in total lysate after 48 h) were examined by immunoblot analysis. Data are presented as mean±s.e.m., *P<0.05.
Interleukin-8 mediates FKBP51-induced growth, clonogenicity and aggressive phenotypes of melanoma cells
To explore the involvement of IL-8 in FKBP51-promoted melanoma pathogenesis, A375SM-NT and FEMX-1-NT cells were treated with IL-8-neutralising antibody or transiently transfected with IL-8-specific siRNAs, whereas A375SM-shFKBP51 and FEMX-1-shFKBP51 cells were treated with rIL-8, and effect on growth and malignant behaviour was examined. In accordance to our in vivo data, we observed ∼3.6-fold and ∼3.4-fold decreased growth in FKBP51-silenced A375SM and FEMX-1 cells, respectively, as compared with that in controls. Furthermore, treatment with IL-8-neutralising antibody also resulted in the significant growth inhibition of A375SM-NT (∼2.2-fold) and FEMX-1-NT (∼1.8-fold) cells (Figure 4A). Interestingly, treatment in A375SM- and FEMX-1-shFKBP51 cells partially abrogated the growth-inhibitory effects of FKBP51 silencing (Figure 4A). Next, we performed plating efficiency assay to monitor growth in the long term. Our data revealed that knockdown of FKBP51 was able to decrease the clonogenic potential of A375SM and FEMX-1 cells by ∼4.7-fold and ∼5.3-fold, respectively, as compared with control cells (Figure 4B). Additionally, inhibition of IL-8 by its neutralising antibody also decreases the clonogenic ability of A375SM-NT (∼2.3-fold) and FEMX-1-NT (∼2.0-fold) cells. Furthermore, cells (A375SM-shFKBP51 and FEMX-1-shFKBP51) treated with rIL-8 significantly enhances the colony formation (Figure 4B). In the next set of experiments, we studied the significance of IL-8 in FKBP51-induced aggressive phenotypes of melanoma cells. Our data demonstrate that FKBP51 silencing diminishes the migration (∼3.3- and ∼4.2-folds; Figure 5A) and invasion (∼6.7- and ∼5.2-folds; Figure 5B) in A375SM and FEMX-1 cells, respectively, as compared with their relevant controls. Inhibition of IL-8 by neutralising antibody (in FKBP51-expressing cells) also decreased the cell migration (∼2.7- and ∼2.3-folds) and invasion (∼2.4- and ∼2.6-folds) (Figure 5A and B). On the other hand, cells treated with rIL-8, partially neutralised the effects of FKBP51 silencing and promoted their migration (∼3.0- and ∼2.8-folds, respectively) and invasion (∼2.3- and ∼1.9-folds, respectively) (Figure 5A and B). Similar to the effects of IL-8-neutralising antibody, we also observed the inhibitory effects of IL-8 silencing on the growth and malignant behaviour of melanoma cells (Supplementary Figure S3). Altogether, our data suggest that FKBP51 regulated melanoma growth and aggressiveness is mediated, at least in part, through IL-8.
FKBP51 promotes melanoma growth by regulating IL-8. (A) For cell growth assay, controls and FKBP51-silenced cells were seeded (5 × 104 per well) in 96-well plate, and growth was monitored after 96 h as described in materials and methods. In parallel, NT cells seeded in 96 wells were treated with control IgG (200 ng ml−1) or IL-8-neutralising antibody (200 ng ml−1) and FKBP51-silenced cells were treated with rIL-8 (10 ng ml−1) and effect on cell growth was determined. (B) For plating efficiency assay, cells were seeded (1 × 103cells per well) in 6-well plates in complete media and allowed to form colonies for 2 weeks. Separately, NT (1 × 103 cells per well) cells were treated with control IgG (200 ng ml−1) or IL-8-neutralising antibody (200 ng ml−1) and FKBP51-silenced (1 × 103 cells per well) cells were treated with rIL-8 (10 ng ml−1). After 2 weeks, colonies were fixed with methanol, stained with crystal violet, photographed and counted using Image analysis software. Bars represent mean±s.e.m., n=3, *P<0.05.
FKBP51-induced malignant potential is mediated through IL-8. Controls and FKBP51/IL-8-silenced cells were seeded on (A) non-coated (for motility assay), or (B) Matrigel-coated (for invasion assay) membranes. Media containing 10% FBS (for controls, FKBP51/IL-8-silenced cells) or rIL-8 (10 ng ml−1, for FKBP51-silenced cells, pretreated with rIL-8) was added as a chemoattractant. Migrated (A) and invaded (B) cells were counted and presented as average number of cells per field±s.e.m., *P<0.05. Photographs are representative of three experiments done in triplicate.
Interleukin-8 mediates the effect of FKBP51 overexpression on angiogenesis
Having observed an association of FKBP51-expressing cells-derived xenograft with enhanced angiogenesis, we next examined whether this effect is also mediated through IL-8. For this, we performed in vitro endothelial (HUVEC) cell proliferation and capillary-like structure formation assays in the presence of CM from FKBP51-expressing and -silenced cells. The data demonstrate a decreased proliferation of HUVEC when grown in CM from A375SM-shFKBP51 (∼5.5-fold) and FEMX-1-shFKBP51 (∼5.8-fold) cells, respectively, as compared with that from control cells (Figure 6A). Treatment of HUVECs with the CM (treated with IL-8-neutralising antibody for 24 h) obtained from A375SM-NT and FEMX-1-NT cells resulted in significant reduction of HUVECs growth (∼2.9- and ∼2.5-folds, respectively) (Figure 6A). In a separate experiment, treatment of HUVECs with rIL-8 promoted their proliferation by ∼6.3-fold (Figure 6B). These effects were further substantiated by conducting CLS formation assay. Cells seeded on Matrigel in the presence of CM (from A375SM-NT and FEMX-1-NT), exhibited extensive CLS formation (∼17–20 CLS/field), while very less CLS formation was observed when HUVECs were treated with CM from FKBP51-silenced cells (Figure 6C). In order to validate a role of IL-8 in FKBP51-induced CLS formation of HUVEC, we treated HUVEC with CM obtained from FKBP51-expressing cells that had been pre-treated with IL-8-neutralising antibody. Data demonstrate that neutralising antibody to IL-8 significantly inhibited the CLS formation (Figure 6C) of HUVECs. Similarly, we also observe significant reduction in the HUVEC proliferation as well as CLS formation upon treatment with CM of IL-8 silenced melanoma cells (Supplementary Figure S4A and B). On the contrary, higher number (∼20 CLS per field) of CLS is observed upon treatment of HUVEC with rIL-8 (Figure 6D). Thus, these data support the involvement of IL-8 in mediating the effect of FKBP51 on angiogenesis in melanoma.
FKBP51 silencing decreases in vitro angiogenesis through inhibiting IL-8. For HUVEC proliferation assay (A and B), HUVECs were incubated with CM obtained from NT, FKBP51-silenced cells for 48 h and effect on cell proliferation was monitored using WST-1 assay kit. Separately, CM from FKBP51-NT cells was treated with IL-8-neutralising antibody (200 ng ml−1) for 24 h. Subsequently, effect on HUVECs growth was examined. In a parallel study, HUVECs were cultured in low serum (2% serum) media or low serum media containing rIL-8 (10 ng ml−1), and cell proliferation was examined after 48 h as described above. For in vitro angiogenesis assay (C and D), cells were plated on Matrigel-coated 96-well plates and incubated with CM from controls, FKBP51-silenced/IL-8-neutralising antibody (200 ng ml−1)-treated cells. After 16 h of incubation, CLS formation was observed and photographed. In a separate experiment, HUVECs were plated on Matrigel-coated 96-well plates in 2% serum containing media or media (with 2% serum) containing rIL-8 (10 ng ml−1), and CLS formation was examined after 16 h. Data are presented as mean±s.e.m., *P<0.05.
Discussion
In the present study, we demonstrated that inhibition of FKBP51 significantly reduced the tumourigenicity, metastatic potential, neutrophil infiltration and angiogenesis of melanoma cells. Moreover, we demonstrated that FKBP51 expression correlated with IL-8 level in tumour xenograft developed from FKBP51-silenced melanoma cells as well as in cell lines. Furthermore, mechanistic and functional studies revealed that FKBP51-induced IL-8 expression is mediated through NF-κB activation, and it serves as a key mediator in FKBP51-induced melanoma growth, metastasis, immune cell infiltration and angiogenesis.
A role of FKBP51 in melanoma pathogenesis has been suggested in recent studies (Romano et al, 2004, 2013, 2014). In the same line, this study provided direct evidence for a functional role of FKBP51 in melanoma growth and metastasis. Moreover, in additional novel findings, FKBP51 overexpression was associated with enhanced neutrophil infiltration and angiogenesis within the tumour xenograft. This is quite interesting as these phenotypes are significant for progressive growth of tumour. New blood vessel formation is required to support the increasing need of food supply to the growing tumour mass as well as to facilitate their haematogenous spread to distant organs (Li et al, 2003; Autiero et al, 2005; Sadanandam et al, 2010). Similarly, in the last decade, significant attention has been focused on tumour-associated neutrophils that are recruited into the tumour mass to support cancer progression. During the extravasation to the tumour site, neutrophils remodel the extracellular matrix by releasing several proteases, heparinase and various enzymes (Mollinedo et al, 1999). These released factors can act as growth stimulators and/or chemoattractants for the tumour and/or endothelial cells and thus assist in the metastatic processes (De Larco et al, 2004; Tazzyman et al, 2009; Spicer et al, 2012). Accordingly, higher level of neutrophil infiltration has been shown to be associated with poor survival, tumour-grade and aggressiveness in a variety of cancers (Fossati et al, 1999; Reid et al, 2011). In that regard, our data on the effect of FKBP51 on vessel density and neutrophil infiltration add a novel insight into its multiple pathological involvements in melanoma growth and metastasis.
For the recruitment of neutrophils to the tumour site, they must leave the general circulation and transmigrate across the vasculature (Tazzyman et al, 2009). It is well known that chemokines may stimulate the transmigration of neutrophils, and IL-8 is one of the chemokine, which acts as a major neutrophil chemoattractant (Huber et al, 1991). Moreover, it has been demonstrated that IL-8-expressing melanoma cells physically interact with neutrophils to facilitate their lung metastasis (Huh et al, 2010). Interleukin-8 has been demonstrated to positively influence the endothelial cells as well as tumour growth through autocrine and paracrine signalling (Li et al, 2003; Singh et al, 2011). Overexpression of IL-8 significantly induced the primary tumour growth and lung metastasis in melanoma (Wu et al, 2012). Moreover, its role in modulating growth and invasiveness of oestrogen receptor-negative breast cancer cells (Yao et al, 2007) and prostate cancer cells (Ma et al, 2009) has also been reported. Similarly, significant data exist to support a role of IL-8 in angiogenesis. Overexpression of IL-8 or exogenous addition of IL-8 was shown to promote endothelial cell survival, proliferation and induced capillary formation (Li et al, 2003; Wu et al, 2012). Interleukin-8 exerted these effects upon binding to CXCR1/CXCR2 receptors on endothelial cells and silencing of these receptors suppressed angiogenic phenotypes (Singh et al, 2011). More importantly, in our previous study, we provided direct evidence for a role of IL-8-CXCR2 in melanoma growth, metastasis and angiogenesis using a CXCR2 knockout mouse model (Singh et al, 2009a).
Interleukin-8 is constitutively expressed in melanoma, however, it has remained largely unknown what factors elicit its upregulation in melanoma cells. Like many other cytokines and chemokines, its expression can be regulated through a variety of mechanisms (Roebuck, 1999; Xie, 2001; Sakamoto et al, 2003). Here, our studies revealed that FKBP51 is one of the dysregulated inducers of IL-8 expression in melanoma cells. Moreover, we identified that IL-8 regulation by FKBP51 was mediated through NF-κB activation. NF-κB is a transcription factor that is aberrantly activated in several malignancies and there are indications that its constitutive activation may involve positive feedback mechanisms (Lin et al, 2010). For example, CXCR4 is known to be a downstream target gene of NF-κB (Bist et al, 2011), whereas activation of CXCR4 itself is shown to promote NF-κB nuclear translocation and enhanced transcriptional activity of NF-κB-responsive promoter (Kukreja et al, 2005). Interleukin-8 is also reported to be one of the target genes of NF-κB (Kunsch and Rosen, 1993) and is also known to activate it in a positive feed-back mechanism (Manna and Ramesh, 2005). Therefore, FKBP51-induced enhanced production of IL-8 may elicit multiple autocrine and/or paracrine signalling pathways to promote melanoma pathogenesis.
In conclusion, our study have shown that FKBP51 regulates IL-8 through NF-κB activation, which then contributes to enhanced growth, metastatic potential and angiogenesis in melanoma. These novel findings strengthen the pathological significance of FKBP51 in melanoma and are quite significant from the perspective of molecular pathogenesis of melanoma. Together, our data shed new light into involved cancer mechanisms that could be targeted for development of effective melanoma therapy.
Accession codes
Change history
26 May 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Autiero M, De SF, Claes F, Carmeliet P (2005) Role of neural guidance signals in blood vessel navigation. Cardiovasc Res 65 (3): 629–638.
Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, McClellan S, Carter JE, Singh AP (2014) CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget 5 (22): 11490–11500.
Bist P, Leow SC, Phua QH, Shu S, Zhuang Q, Loh WT, Nguyen TH, Zhou JB, Hooi SC, Lim LH (2011) Annexin-1 interacts with NEMO and RIP1 to constitutively activate IKK complex and NF-kappaB: implication in breast cancer metastasis. Oncogene 30 (28): 3174–3185.
Bracher A, Kozany C, Hahle A, Wild P, Zacharias M, Hausch F (2013) Crystal structures of the free and ligand-bound FK1-FK2 domain segment of FKBP52 reveal a flexible inter-domain hinge. J Mol Biol 425 (22): 4134–4144.
De Larco JE, Wuertz BR, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10 (15): 4895–4900.
Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML (1999) Neutrophil infiltration into human gliomas. Acta Neuropathol 98 (4): 349–354.
Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A, Zupi G, Del BD (2009) Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer 45 (14): 2618–2627.
Galat A (1993) Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem 216 (3): 689–707.
Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M (2002) Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol 161 (1): 125–134.
Huber AR, Kunkel SL, Todd RF III, Weiss SJ (1991) Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254 (5028): 99–102.
Huh SJ, Liang S, Sharma A, Dong C, Robertson GP (2010) Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70 (14): 6071–6082.
Jiang W, Cazacu S, Xiang C, Zenklusen JC, Fine HA, Berens M, Armstrong B, Brodie C, Mikkelsen T (2008) FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-kappaB signaling pathway. Neoplasia 10 (3): 235–243.
Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC (2005) Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res 65 (21): 9891–9898.
Kunsch C, Rosen CA (1993) NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13 (10): 6137–6146.
Li A, Dubey S, Varney ML, Dave BJ, Singh RK (2003) IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170 (6): 3369–3376.
Lin Y, Bai L, Chen W, Xu S (2010) The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets 14 (1): 45–55.
Ma J, Ren Z, Ma Y, Xu L, Zhao Y, Zheng C, Fang Y, Xue T, Sun B, Xiao W (2009) Targeted knockdown of EGR-1 inhibits IL-8 production and IL-8-mediated invasion of prostate cancer cells through suppressing EGR-1/NF-kappaB synergy. J Biol Chem 284 (50): 34600–34606.
Manna SK, Ramesh GT (2005) Interleukin-8 induces nuclear transcription factor-kappaB through a TRAF6-dependent pathway. J Biol Chem 280 (8): 7010–7021.
Mollinedo F, Borregaard N, Boxer LA (1999) Novel trends in neutrophil structure, function and development. Immunol Today 20 (12): 535–537.
Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, Altinel D, Adsay V (2011) Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol 24 (12): 1612–1619.
Rodic S, Mihalcioiu C, Saleh RR (2014) Detection methods of circulating tumor cells in cutaneous melanoma: a systematic review. Crit Rev Oncol Hematol 91 (1): 74–92.
Roebuck KA (1999) Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 19 (5): 429–438.
Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S (2004) Rapamycin inhibits doxorubicin-induced NF-kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer 40 (18): 2829–2836.
Romano S, D'Angelillo A, D'Arrigo P, Staibano S, Greco A, Brunetti A, Scalvenzi M, Bisogni R, Scala I, Romano MF (2014) FKBP51 increases the tumour-promoter potential of TGF-beta. Clin Transl Med 3 (1): 1–3.
Romano S, D'Angelillo A, Pacelli R, Staibano S, De LE, Bisogni R, Eskelinen EL, Mascolo M, Cali G, Arra C, Romano MF (2010) Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ 17 (1): 145–157.
Romano S, Mallardo M, Romano MF (2011) FKBP51 and the NF-kappaB regulatory pathway in cancer. Curr Opin Pharmacol 11 (4): 288–293.
Romano S, Staibano S, Greco A, Brunetti A, Nappo G, Ilardi G, Martinelli R, Sorrentino A, Di PA, Mascolo M, Bisogni R, Scalvenzi M, Alfano B, Romano MF (2013) FK506 binding protein 51 positively regulates melanoma stemness and metastatic potential. Cell Death Dis 4: e578.
Sadanandam A, Rosenbaugh EG, Singh S, Varney M, Singh RK (2010) Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc Res 79 (1): 1–9.
Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T, Terakawa N (2003) Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab 88 (2): 730–735.
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65 (1): 5–29.
Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J (2003) Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A 100 (3): 868–873.
Singh AP, Arora S, Bhardwaj A, Srivastava SK, Kadakia MP, Wang B, Grizzle WE, Owen LB, Singh S (2012) CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor kappaB: implications for bidirectional tumor-stromal interactions. J Biol Chem 287 (46): 39115–39124.
Singh S, Nannuru KC, Sadanandam A, Varney ML, Singh RK (2009a) CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer 19 (10): 1638–1646.
Singh S, Singh AP, Sharma B, Owen LB, Singh RK (2010) CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol 6 (1): 111–116.
Singh S, Varney M, Singh RK (2009b) Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res 69 (2): 411–415.
Singh S, Wu S, Varney M, Singh AP, Singh RK (2011) CXCR1 and CXCR2 silencing modulates CXCL8-dependent endothelial cell proliferation, migration and capillary-like structure formation. Microvasc Res 82 (3): 318–325.
Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE (2012) Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72 (16): 3919–3927.
Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB (2011) FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab 22 (12): 481–490.
Sun V, Zhou WB, Nosrati M, Majid S, Thummala S, de SD, Bezrookove V, de FS, Chun L, Schadendorf D, Debs R, Kashani-Sabet M, Dar AA (2015) Antitumor activity of miR-1280 in melanoma by regulation of Src. Mol Ther 23 (1): 71–78.
Tazzyman S, Lewis CE, Murdoch C (2009) Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol 90 (3): 222–231.
Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S (2014) p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-kappaB pathway. Oncotarget 5 (18): 8778–8789.
Varney ML, Johansson SL, Singh RK (2006) Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol 125 (2): 209–216.
Wang JM, Taraboletti G, Matsushima K, Van DJ, Mantovani A (1990) Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun 169 (1): 165–170.
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14 (21): 6735–6741.
Wu S, Singh S, Varney ML, Kindle S, Singh RK (2012) Modulation of CXCL-8 expression in human melanoma cells regulates tumor growth, angiogenesis, invasion, and metastasis. Cancer Med 1 (3): 306–317.
Xie K (2001) Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 12 (4): 375–391.
Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM (2007) Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer 121 (9): 1949–1957.
Acknowledgements
We would like to acknowledge the funding support from NIH/NCI [CA169829, CA186233 (to SS) and CA167137, CA175772, CA185490 (to APS)] and USAMCI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Srivastava, S., Bhardwaj, A., Arora, S. et al. Interleukin-8 is a key mediator of FKBP51-induced melanoma growth, angiogenesis and metastasis. Br J Cancer 112, 1772–1781 (2015). https://doi.org/10.1038/bjc.2015.154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.154
Keywords
This article is cited by
-
FKBP51 plays an essential role in Akt ubiquitination that requires Hsp90 and PHLPP
Cell Death & Disease (2023)
-
FKBP51 promotes invasion and migration by increasing the autophagic degradation of TIMP3 in clear cell renal cell carcinoma
Cell Death & Disease (2021)
-
FKBP9 promotes the malignant behavior of glioblastoma cells and confers resistance to endoplasmic reticulum stress inducers
Journal of Experimental & Clinical Cancer Research (2020)
-
The biomarker HE4 (WFDC2) promotes a pro-angiogenic and immunosuppressive tumor microenvironment via regulation of STAT3 target genes
Scientific Reports (2020)
-
Targeting polyamine biosynthetic pathway through RNAi causes the abrogation of MCF 7 breast cancer cell line
Tumor Biology (2016)