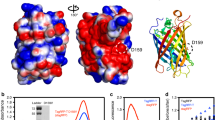
Abstract
Recently, several groups have developed green fluorescent protein (GFP)-based Ca2+ probes. When applied in cells, however, these probes are difficult to use because of a low signal-to-noise ratio. Here we report the development of a high-affinity Ca2+ probe composed of a single GFP (named G-CaMP). G-CaMP showed an apparent Kd for Ca2+ of 235 nM. Association kinetics of Ca2+ binding were faster at higher Ca2+ concentrations, with time constants decreasing from 230 ms at 0.2 μM Ca2+ to 2.5 ms at 1 μM Ca2+. Dissociation kinetics (τ ∼200 ms) are independent of Ca2+ concentrations. In HEK-293 cells and mouse myotubes expressing G-CaMP, large fluorescent changes were observed in response to application of drugs or electrical stimulations. G-CaMP will be a useful tool for visualizing intracellular Ca2+ in living cells. Mutational analysis, together with previous structural information, suggests the residues that may alter the fluorescence of GFP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Inouye, S. et al. Cloning and sequence analysis of cDNA for the luminescent protein aequorin. Proc. Natl. Acad. Sci. USA 82, 3154–3158 (1985).
Prasher, D.C., Eckenrode, V.K., Ward, W.W., Prendergast, F.G. & Cormier, M.J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111, 229–233 (1992).
Miyawaki, A. et al. Fluorescent indicators for Ca 2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Miyawaki, A., Griesbeck, O., Heim, R. & Tsien, R.Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. USA 96, 2135–2140 (1999).
Romoser, V.A., Hinkle, P.M. & Persechini, A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. J. Biol. Chem. 272, 13270–13274 (1997).
Persechini, A., Lynch, J.A. & Romoser, V.A. Novel fluorescent indicator proteins for monitoring free intracellular Ca2+. Cell Calcium 22, 209–216 (1997).
Baird, G.S., Zacharias, D.A. & Tsien, R.Y. Circular permutation and receptor insertion within green fluorescent proteins. Proc. Natl. Acad. Sci. USA 96, 11241–11246 (1999).
Baubet, V. et al. Chimeric green fluorescent protein–aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc. Natl. Acad. Sci. USA 97, 7260–7265 (2000).
Allen, G.J. et al. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19, 735–747 (1999).
Emmanouilidou, E. et al. Imaging Ca2+ concentration changes at the secretory vesicle surface with a recombinant targeted cameleon. Curr. Biol. 9, 915–918 (1999).
Fan, G.Y. et al. Video-rate scanning two-photon excitation fluorescence microscopy and ratio imaging with cameleons. Biophys. J. 76, 2412–2420 (1999).
Jaconi, M. et al. Inositol 1,4,5-trisphosphate directs Ca2+ flow between mitochondria and the endoplasmic/sarcoplasmic reticulum: a role in regulating cardiac autonomic Ca2+ spiking. Mol. Biol. Cell 11, 1845–1858 (2000).
Foyouzi-Youssefi, R. et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 97, 5723–5728 (2000).
Yu, R. & Hinkle, P.M. Rapid turnover of calcium in the endoplasmic reticulum during signaling: studies with cameleon calcium indicators. J. Biol. Chem. 275, 23648–23653 (2000).
Kerr, R. et al. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26, 583–594 (2000).
Allen, G.J. et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289, 2338–2342 (2000).
Rhoads, A.R. & Friedberg, F. Sequence motifs for calmodulin recognition. FASEB J. 11, 331–340 (1997).
Mori, M. et al. Novel interaction of the voltage-dependent sodium channel (VDSC) with calmodulin: does VDSC acquire calmodulin-mediated Ca2+-sensitivity? Biochemistry 39, 1316–1323 (2000).
Bischof, G., Serwold, T.F. & Machen, T.E. Does nitric oxide regulate capacitative Ca influx in HEK 293 cells? Cell Calcium 21, 135–142 (1997).
Yang, F., Moss, L.G. & Phillips, G.N. Jr. The molecular structure of green fluorescent protein. Nat. Biotechnol. 14, 1246–1251 (1996).
Dickson, R.M., Cubitt, A.B., Tsien, R.Y. & Moerner, W.E. On/off blinking and switching behavior of single molecules of green fluorescent protein. Nature 388, 355–358 (1997).
Okada, T. et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. J. Biol. Chem. 274, 27359–27370 (1999).
Cormack, B.P., Valdivia, R.H. & Falkow, S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173, 33–38 (1996).
Nakai, J. et al. Functional nonequality of the cardiac and skeletal ryanodine receptors. Proc. Natl. Acad. Sci. USA 94, 1019–1022 (1997).
Bers, D.M., Patton, C.W. & Nuccitelli, R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 40, 3–29 (1994).
James-Kracke, M.R. Quick and accurate method to convert BCECF fluorescence to pHi: calibration in three different types of cell preparations. J. Cell. Physiol. 151, 596–603 (1992).
Tanabe, T., Beam, K.G., Powell, J.A. & Numa, S. Restoration of excitation–contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 336, 134–139 (1988).
Acknowledgements
We thank Masayuki Mori for the rat calmodulin cDNA and Michiyo Murata and Mitsutoshi Ono for technical assistance. We also thank Toshihiko Nagamura and Tatsuo Nakagawa of Unisoku Co., Ltd. for technical assistance. The work was supported by grants from the Ministry of Education, Science, Sports and Culture, by “the Research for the Future Program” of the JSPS, and by the JSPS Research Fellowships for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol 19, 137–141 (2001). https://doi.org/10.1038/84397
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84397
This article is cited by
-
Perspective of Calcium Imaging Technology Applied to Acupuncture Research
Chinese Journal of Integrative Medicine (2024)
-
Highly efficient Agrobacterium rhizogenes-mediated transformation for functional analysis in woodland strawberry
Plant Methods (2023)
-
A closer look at jGCaMP8
Lab Animal (2023)
-
Chemogenetic inhibition of subicular seizure-activated neurons alleviates cognitive deficit in male mouse epilepsy model
Acta Pharmacologica Sinica (2023)
-
Integrating operant behavior and fiber photometry with the open-source python library Pyfiber
Scientific Reports (2023)