Abstract
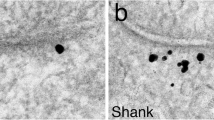
Phosphorylation of synapsin I by CaMKII has been reported to mobilize synaptic vesicles from the reserve pool. In the present study, the distributions of α-CaMKII and of synapsin I were compared in synaptic boutons of unstimulated and stimulated hippocampal neurons in culture by immunogold electron microscopy. CaMKII and synapsin I are located in separate domains in presynaptic terminals of unstimulated neurons. Label for α -CaMKII typically surrounds synaptic vesicle clusters and is absent from the inside of the cluster in control synapses. In contrast, intense labeling for synapsin I is found within the vesicle clusters. Following 2 minutes of depolarization in high K+, synaptic vesicles decluster and CaMKII label disperses and mingles with vesicles and synapsin I. These results indicate that, under resting conditions, CaMKII has limited access to the synapsin I in synaptic vesicle clusters. The peripheral distribution of CaMKII around vesicle clusters suggests that CaMKII-mediated declustering progresses from the periphery towards the center, with the depth of penetration into the synaptic vesicle cluster depending on the duration of CaMKII activation. Depolarization also promotes a significant increase in CaMKII immunolabel near the presynaptic active zone. Activity-induced redistribution of CaMKII leaves it in a position to facilitate phosphorylation of additional presynaptic proteins regulating neurotransmitter release.






Similar content being viewed by others
References
Barria, A., Muller, D., Derkach, V., Griffith, L. C., and Soderling, T. R. (1997). Regulatory phosphorylation of AMPA-type glutamate receptors by CaMKII during long-term potentiation. Science 276, 2042–2045
Chi, P., Greengard, P., and Ryan, T. A. (2001). Synapsin dispersion and reclustering during synaptic activity. Nat. Neurosci. 4, 1187–1193
Chi, P., Greengard, P., and Ryan, T. A. (2003). Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 38, 69–78
Colbran, R. J. (2004a) Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J. Neurosci. 24, 8404–8409
Colbran, R. J. (2004b). Targeting of calcium/calmodulin-dependent protein kinase II. Biochem. J. 378, 1–16
Dosemeci, A., Reese, T. S., Petersen, J., and Tao-Cheng, J. H. (2000). A novel particulate form of Ca(2+)/calmodulin-dependent protein kinase II in neurons. J. Neurosci. 20, 3076–3084
Dosemeci, A., Tao-Cheng, J. H., Vinade, L., Winters, C. A., Pozzo-Miller, L., and Reese, T. S. (2001). Glutamate-induced transient modification of the postsynaptic density. Proc. Natl. Acad. Sci. USA 98, 10428–10432
Dosemeci, A., Vinade, L., Winters, C. A., Reese, T. S., and Tao-Cheng, J. -H. (2002). Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. J. Neurocytol. 31, 605–612
Dunaevsky, A., and Connor, E. A. (2000). F-actin is concentrated in nonrelease domains at frog neuromuscular junctions. J. Neurosci. 20, 6007–6012
Gitler, D., Takagishi, Y., Feng, J., Ren, Y., Rodriguiz, R. M., Wetsel, W. C., Greengard, P., and Augustine, G. J. (2004). Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J. Neurosci. 24, 11368–11380
Hagiwara, A., Fukazawa, Y., Deguchi-Tawarada, M., Ohtsuka, T., and Shigemoto, R. (2005). Differential distribution of release-related proteins in the hippocampal CA3 area as revealed by freeze-fracture replica labeling. J. Comp. Neurol. 489, 195–216
Hilfiker, S., Pieribone, V. A., Czernik, A. J., Kao, H. T., Augustine, G. J., and Greengard, P. (1999). Synapsins as regulators of neurotransmitter release. Philos. Trans. R Soc. Lond. B Biol. Sci. 354, 269–279
Landis, D. M. D., Hall, A. K., Weinstein, L. A., and Reese, T. S. (1988). The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron 1, 201–209.
Lisman, J., Schulman, H., and Cline H. (2002). The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3, 175–190
Liu, X. B., and Jones, E. G. (1996). Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc. Natl. Acad. Sci. USA 93, 7332–7336
Lu, Z., McLaren, R. S., Winters, C. A., and Ralston, E. (1998). Ribosome association contributes to restricting mRNAs to the cell body of hippocampal neurons. Mol. Cell. Neurosci. 12, 363–375
Malinow, R., Schulman, H., and Tsien, R. W. (1989). Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866
Mammen, A. L., Kameyama, K., Roche, K. W., and Huganir, R. L. (1997). Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 272, 32528–32533
Nayak, A. S., Moore, C. I., and Browning, M. D. (1996). Ca2+/calmodulin-dependent protein kinase II phosphorylation of the presynaptic protein synapsin I is persistently increased during long-term potentiation. Proc. Natl. Acad. Sci. USA 93, 15451–15456
Ninan, I., and Arancio, O. (2004). Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron 42, 129–141
Ohyama, A., Hosaka, K., Komiya, Y., Akagawa, K., Yamauchi, E., Taniguchi, H., Sasagawa, N., Kumakura, K., Mochida, S., Yamauchi, T., and Igarashi, M. (2002). Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J. Neurosci. 22, 3342–3351
Otmakhov, N., Griffith, L. C., and Lisman, J. E. (1997). Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation. J. Neurosci. 17, 5357–5365
Pieribone, V. A., Shupliakov, O., Brodin, L., Hilfiker-Rothenfluh, S., Czernik, A. J., and Greengard, P. (1995). Distinct pools of synaptic vesicles in neurotransmitter release. Nature 375, 493–497
Sankaranarayanan, S., Atluri, P. P., and Ryan, T. A. (2003). Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 6, 127–135.
Sesack, S. R. and Snyder, C. L. (1995). Cellular and subcellular localization of syntaxin-like immunoreactivity in the rat striatum and cortex. Neuroscience 67, 993–1007
Shen, K. and Meyer, T. (1999). Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284, 162–166
Silva, A. J., Stevens, C. F., Tonegawa, S., and Wang, Y. (1992). Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science 257, 201–206
Snyder, D. A., Kelly, M. L., Woodbury, D. J. (2006). SNARE complex regulation by phosphorylation. Cell. Biochem. Biophys. 45, 111–123
Tanner, V. A., Ploug, T., and Tao-Cheng, J. H. (1996). Subcellular localization of SV2 and other secretory vesicle components in PC12 cells by an efficient method of preembedding EM immunocytochemistry for cell cultures. J. Histochem. Cytochem. 44, 1481–1488
Tao-Cheng, J.-H., Du, J., McBain, C. J. (2000). SNAP-25 is polarized to axons and abundant throughout the axolemma: an immunogold study. J. Neurocytol. 29, 67–77.
Tao-Cheng, J. H. (2006). Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience 141, 1217–1224
Turner, K. M., Burgoyne, R. D., and Morgan, A. (1999). Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 22, 459–464
Verona, M., Zanotti, S., Schafer, T., Racagni, G., and Popoli, M. (2000). Changes of synaptotagmin interaction with t-SNARE proteins in fsvitro after calcium/calmodulin-dependent phosphorylation. J. Neurochem. 74, 209–221
Yokoyama, C. T., Sheng, Z. H., and Catterall, W. A. (1997). Phosphorylation of the synaptic protein interaction site on N-type calcium channels inhibits interactions with SNARE proteins. J. Neurosci. 17, 6929–6938
Acknowledgments
We thank Virginia Crocker and Rita Azzam for EM technical support. This research was supported by the Intramural Research Program of the NIH, NINDS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao-Cheng, JH., Dosemeci, A., Winters, C. et al. Changes in the distribution of calcium calmodulin-dependent protein kinase II at the presynaptic bouton after depolarization. Brain Cell Bio 35, 117–124 (2006). https://doi.org/10.1007/s11068-007-9012-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11068-007-9012-5