Abstract
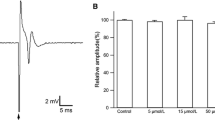
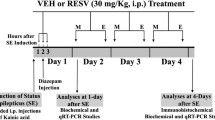
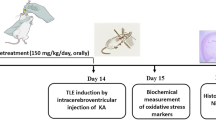
Resveratrol (Res) is a phytoalexin produced naturally by several plants, which has multi functional effects such as neuroprotection, anti-inflammatory, and anti-cancer. The present study was to evaluate a possible anti-epileptic effect of Res against kainate-induced temporal lobe epilepsy (TLE) in rat. We performed behavior monitoring, intracranial electroencepholography (IEEG) recording, histological analysis, and Western blotting to evaluate the anti-epilepsy effect of Res in kainate-induced epileptic rats. Res decreased the frequency of spontaneous seizures and inhibited the epileptiform discharges. Moreover, Res could protect neurons against kainate-induced neuronal cell death in CA1 and CA3a regions and depressed mossy fiber sprouting, which are general histological characteristics both in TLE patients and animal models. Western blot revealed that the expression level of kainate receptors (KARs) in hippocampus was reduced in Res-administrated rats compared to that in epileptic ones. These results suggest that Res is a potent anti-epilepsy agent, which protects against epileptogenesis and progression of the kainate-induced TLE animal.



Similar content being viewed by others
References
Ben-Ari Y, Represa A (1990) Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci 13:312–318. doi:10.1016/0166-2236(90)90135-W
Nadler JV (2003) The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res 28:1649–1658. doi:10.1023/A:1026004904199
Shetty AK, Zaman V, Hattiangady B (2005) Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci 25:8391–8401. doi:10.1523/JNEUROSCI.1538-05.2005
Mathern GW, Babb TL, Leite JP et al (1996) The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res 26:151–161. doi:10.1016/S0920-1211(96)00052-6
Buckmaster PS, Zhang GF, Yamawaki R (2002) Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci 22:6650–6658
Epsztein J, Represa A, Jorquera I et al (2005) Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats. J Neurosci 25:8229–8239. doi:10.1523/JNEUROSCI.1469-05.2005
Represa A, Tremblay E, Ben-Ari Y (1987) Kainate binding sites in the hippocampal mossy fibers: localization and plasticity. Neuroscience 20:739–748. doi:10.1016/0306-4522(87)90237-5
Ben-Ari Y (1985) Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14:375–403. doi:10.1016/0306-4522(85)90299-4
Das S, Das DK (2007) Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6:168–173. doi:10.2174/187152807781696464
Sun W, Wang W, Kim J et al (2008) Anti-cancer effect of resveratrol is associated with induction of apoptosis via a mitochondrial pathway alignment. Adv Exp Med Biol 614:179–186. doi:10.1007/978-0-387-74911-2_21
Gao ZB, Chen XQ, Hu GY (2006) Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res 1111:41–47. doi:10.1016/j.brainres.2006.06.096
Wu Z, Xu Q, Qian RB et al (2008) Temporal lobe epilepsy animal model established by stereotaxic microinjection of kainic acid. Neural Regen Res 3:436–440
Racine R, Okujava V, Chipashvili S (1972) Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol 32:295–299. doi:10.1016/0013-4694(72)90178-2
Chen Q, He S, Hu XL et al (2007) Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci 27:542–552. doi:10.1523/JNEUROSCI.3607-06.2007
Braga MF, Aroniadou-Anderjaska V, Xie J et al (2003) Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci 23:442–452
Wisden W, Seeburg PH (1993) A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci 13:3582–3598
Bureau I, Bischoff S, Heinemann SF et al (1999) Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci 19:653–663
Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci 23:580–587. doi:10.1016/S0166-2236(00)01659-3
Mulle C, Sailer A, Perez-Otano I et al (1998) Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392:601–605. doi:10.1038/33408
Fisahn A, Contractor A, Traub RD et al (2004) Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci 24:9658–9668. doi:10.1523/JNEUROSCI.2973-04.2004
Zhang X, Cui SS, Wallace AE et al (2002) Relations between brain pathology and temporal lobe epilepsy. J Neurosci 22:6052–6061
Shetty AK, Hattiangady B (2007) Restoration of calbindin after fetal hippocampal CA3 cell grafting into the injured hippocampus in a rat model of temporal lobe epilepsy. Hippocampus 17:943–956. doi:10.1002/hipo.20311
Cheng Y, Sun AY (1994) Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem Res 19:1557–1564. doi:10.1007/BF00969006
Sun AY, Cheng Y, Bu Q et al (1992) The biochemical mechanisms of the excitotoxicity of kainic acid. Free radical formation. Mol Chem Neuropathol 17:51–63
Lewen A, Matz P, Chan PH (2000) Free radical pathways in CNS injury. J Neurotrauma 17:871–890
Monaghan DT, Cotman CW (1982) The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res 252:91–100. doi:10.1016/0006-8993(82)90981-7
Han YS, Zheng WH, Bastianetto S et al (2004) Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol 141:997–1005. doi:10.1038/sj.bjp.0705688
Gupta YK, Briyal S, Chaudhary G (2002) Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol Biochem Behav 71:245–249. doi:10.1016/S0091-3057(01)00663-3
Wang Q, Yu S, Simonyi A et al (2004) Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res 29:2105–2112. doi:10.1007/s11064-004-6883-z
Kim HC, Jhoo WK, Bing G et al (2000) Phenidone prevents kainate-induced neurotoxicity via antioxidant mechanisms. Brain Res 874:15–23. doi:10.1016/S0006-8993(00)02560-9
Sumanont Y, Murakami Y, Tohda M et al (2006) Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci 78:1884–1891. doi:10.1016/j.lfs.2005.08.028
Wisden W, Seeburg PH (1993) Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol 3:291–298. doi:10.1016/0959-4388(93)90120-N
Westbrook GL, Lothman EW (1983) Cellular and synaptic basis of kainic acid-induced hippocampal epileptiform activity. Brain Res 273:97–109. doi:10.1016/0006-8993(83)91098-3
Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17:31–108. doi:10.1146/annurev.ne.17.030194.000335
Vignes M, Collingridge GL (1997) The synaptic activation of kainate receptors. Nature 388:179–182. doi:10.1038/40639
Cossart R, Epsztein J, Tyzio R et al (2002) Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35:147–159. doi:10.1016/S0896-6273(02)00753-5
Marchal C, Mulle C (2004) Postnatal maturation of mossy fibre excitatory transmission in mouse CA3 pyramidal cells: a potential role for kainate receptors. J Physiol 561:27–37. doi:10.1113/jphysiol.2004.069922
Crowell JA, Korytko PJ, Morrissey RL et al (2004) Resveratrol-associated renal toxicity. Toxicol Sci 82:614–619. doi:10.1093/toxsci/kfh263
Acknowledgments
We thank Prof. Y. Shen and Mr. D. Huang for technical assistance and Dr. S. R. Wang for critical comments on the manuscript. This study was supported by grants from the Grants for Scientific Research of BSKY (XJ2005006) from Anhui Medical University, the Excellent Talent of Anhui province of China (06043090), the National Century Excellent Talents in University of China (NCET-06-0557) to L. Wang, and Natural Science Foundation of Anhui Province Department of Education (NSFA KJ2007A028) to D. Kong.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The authors Z. Wu and Q. Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, Z., Xu, Q., Zhang, L. et al. Protective Effect of Resveratrol against Kainate-induced Temporal Lobe Epilepsy in Rats. Neurochem Res 34, 1393–1400 (2009). https://doi.org/10.1007/s11064-009-9920-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-9920-0