Abstract
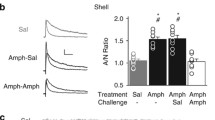
World-wide methamphetamine (meth) use is increasing at a rapid rate; therefore, it has become increasingly important to understand the synaptic changes and neural mechanisms affected by drug exposure. In rodents, 6-h access to contingent meth results in an escalation of drug intake and impaired cognitive sequelae typically associated with changes within the corticostriatal circuitry. There is a dearth of knowledge regarding the underlying physiological changes within this circuit following meth self-administration. We assessed pre- and postsynaptic changes in glutamate transmission in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) following daily 6-h meth self-administration. In the mPFC, meth caused postsynaptic adaptations in ionotropic glutamate receptor distribution and function, expressed as a decrease in AMPA/NMDA ratio. This change was driven by an increase in NMDA receptor currents and an increase in GluN2B surface expression. In the NAc, meth decreased the paired-pulse ratio and increased the frequency of spontaneous excitatory postsynaptic currents with no indication of postsynaptic changes. These changes in mPFC synapses and NAc activity begin to characterize the impact of meth on the corticostriatal circuitry.





Similar content being viewed by others
References
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science (New York, NY) 282:298–300
Beccano-Kelly DA, Kuhlmann N, Tatarnikov I, Volta M, Munsie LN, Chou P, Cao LP, Han H, Tapia L, Farrer MJ, Milnerwood AJ (2014) Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Front Cell Neurosci 8:301
Bonci A, Williams JT (1997) Increased probability of GABA release during withdrawal from morphine. J Neurosci 17:796–803
Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB (2009) Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One 4:e7812
Cull-Candy SG, Leszkiewicz DN (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 255:16
Debanne D, Guérineau NC, Gähwiler BH, Thompson SM (1996) Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol (Lond) Wiley-Blackwell 491:163–176
Gao C, Wolf ME (2007) Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. J Neurosci 27:14275–14285
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669
González B, Rivero-Echeto C, Muñiz JA, Cadet JL, García-Rill E, Urbano FJ, Bisagno V (2015) Methamphetamine blunts Ca2+ currents and excitatory synaptic transmission through D1/5 receptor-mediated mechanisms in the mouse medial prefrontal cortex. Addict Biol 21:589–602
Graves SM, Clark MJ, Traynor JR, Hu XT, Napier TC (2015) Nucleus accumbens shell excitability is decreased by methamphetamine self-administration and increased by 5-HT2C receptor inverse agonism and agonism. Neuropharmacology 89:113–121
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Jedynak J, Hearing M, Ingebretson A, Ebner SR, Kelly M, Fischer RA, Kourrich S, Thomas MJ (2016) Cocaine and amphetamine induce overlapping but distinct patterns of AMPAR plasticity in nucleus accumbens medium spiny neurons. Neuropsychopharmacology 41:464–476
Johnson-Davis KL, Fleckenstein AE, Wilkins DG (2003) The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol 482:151–154
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nature 10:561–572
Kourrich S, Calu DJ, Bonci A (2015) Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci 16:173–184
Kumar SS, Huguenard JR (2003) Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. J Neurosci 23:10074–10083
Lee BR, Dong Y (2011) Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology 61:1060–1069
Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y (2015) Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci 35:8232–8244
Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE (2012) Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology 37:707–722
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME (2014) Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 17:73–80
Luthi A, Luscher C (2014) Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci 17:1635–1643
Mark KA, Quinton MS, Russek SJ, Yamamoto BK (2007) Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci 27:6823–6831
Nestler EJ (2001) Molecular neurobiology of addiction. Am J Addict 10:201–217
O’Neill RA, Bhamidipati A, Bi X, Deb-Basu D, Cahill L, Ferrante J, Gentalen E, Glazer M, Gossett J, Hacker K, Kirby C, Knittle J, Loder R, Mastroieni C, Maclaren M, Mills T, Nguyen U, Parker N, Rice A, Roach D, Suich D, Voehringer D, Voss K, Yang J, Yang T, Vander Horn PB (2006) Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc Natl Acad Sci USA 103:16153–16158
Parsegian A, Glen WB Jr, Lavin A, See RE (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry 69:253–259
Peters J, Scofield MD, Ghee SM, Heinsbroek JA, Reichel CM (2015) Perirhinal cortex mGlu5 receptor activation reduces relapse to methamphetamine seeking by restoring novelty salience. Neuropsychopharmacology 41:1477–1485
Regehr WG (2012) Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol 4(7):a005702
Reichel CM, Chan CH, Ghee SM, See RE (2012) Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology 223:371–380
Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, Dolubizno H, Stefanik MT, Murray CH, Sakas C, Wolf ME (2016) AMPA receptor plasticity in accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol Psychiatry. doi:10.1016/j.biopsych.2016.04.003
Schwendt M, Reichel CM, See RE (2012) Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS One 7(3):e34299-10
Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, Reichel CM (2015) Failure to recognize novelty after extended methamphetamine self-administration results from loss of long-term depression in the perirhinal cortex. Neuropsychopharmacology 40:2526–2535
Self DW (2004) Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 47:242–255
Stevens CF, Tsujimoto T (1995) Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA 92:846–849
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405
Acknowledgments
We would like to thank Shannon Ghee and Carole Berini for technical assistance and Dr. John Dinolfo of the Writing Center at the Medical University of South Carolina for editing the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by NIH/NIDA grant R01DA033049 to CMR and Hjarnfonden Brain Foundation of Sweden to DM.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
D. Mishra and J. I. Pena-Bravo contributed equally.
Rights and permissions
About this article
Cite this article
Mishra, D., Pena-Bravo, J.I., Leong, KC. et al. Methamphetamine self-administration modulates glutamate neurophysiology. Brain Struct Funct 222, 2031–2039 (2017). https://doi.org/10.1007/s00429-016-1322-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1322-x