Abstract
Neuronal cytoplasmic inclusions (NCIs) containing phosphorylated TDP-43 (p-TDP-43) are the pathological hallmarks of motor neuron disease/amyotrophic lateral sclerosis (MND/ALS) and FTLD-TDP. The vast majority of NCIs in the brain and spinal cord also label for ubiquitin and p62, however, we have previously reported a subset of TDP-43 proteinopathy patients who have unusual and abundant p62 positive, TDP-43 negative inclusions in the cerebellum and hippocampus. Here we sought to determine whether these cases carry the hexanucleotide repeat expansion in C9orf72. Repeat primer PCR was performed in 36 MND/ALS, FTLD-MND/ALS and FTLD-TDP cases and four controls. Fourteen individuals with the repeat expansion were detected. In all the 14 expansion mutation cases there were abundant globular and star-shaped p62 positive NCIs in the pyramidal cell layer of the hippocampus, the vast majority of which were p-TDP-43 negative. p62 positive NCIs were also abundant in the cerebellar granular and molecular layers in all cases and in Purkinje cells in 12/14 cases but they were only positive for p-TDP-43 in the granular layer of one case. Abundant p62 positive, p-TDP-43 negative neuronal intranuclear inclusions (NIIs) were seen in 12/14 cases in the pyramidal cell layer of the hippocampus and in 6/14 cases in the cerebellar granular layer. This unusual combination of inclusions appears pathognomonic for C9orf72 repeat expansion positive MND/ALS and FTLD-TDP which we believe form a pathologically distinct subset of TDP-43 proteinopathies. Our results suggest that proteins other than TDP-43 are binding p62 and aggregating in response to the mutation which may play a mechanistic role in neurodegeneration.
Similar content being viewed by others
Introduction
Skein-like and globular ubiquitinated inclusions within motor neurons in the anterior horn of the spinal cord were for many years the hallmark pathology of motor neuron disease/amyotrophic lateral sclerosis (MND/ALS) [16, 17]. Similar inclusions were later reported in the cerebral cortex of tau-negative frontotemporal lobar degeneration (FTLD)[11]. The discovery, that the transactive DNA-binding protein (TDP-43) was the major protein within ubiquitinated inclusions in FTLD-U and MND/ALS [2, 25], led to the classification of these disorders as the TDP-43 proteinopathies [18]. Whilst most cases of MND/ALS and FTLD-TDP (as it is now known) are sporadic, a number of genes have been identified that give rise to TDP-43 deposition including, Progranulin (GRN) [4, 19], TARDBP [7, 30], Optineurin (OPTN) [22], Valosin containing protein (VCP) [12, 24], and most recently Ubiquilin 2 (UBQLN2) [9].
We first reported linkage to chromosome 9p 13−22 in a large Dutch kindred with a dominant form of MND/ALS and frontotemporal dementia (FTD) [33], which was subsequently shown to have TDP-43 deposition [7]. Subsequent studies confirmed linkage in familial MND/ALS [23] and genome-wide association studies of sporadic FTD [32] and sporadic MND/ALS [15, 29] narrowed the region to ~240 kb. Very recently, the polymorphic hexanucleotide repeat (GGGGCC) in intron 1 of C9orf72 gene was shown to be dramatically expanded from a normal range of up to 30 repeats to somewhere between 700 and 1,000 in familial and sporadic MND/ALS and FTD [8, 27] The expansion mutation can be rapidly assessed by repeat primer PCR which revealed that approximately 46% of Finnish familial MND/ALS cases [27] and approximately 23% of familial MND/ALS and 11.7% of familial FTD in North America [8] carried the expanded repeat, making it the most common gene defect in MND/ALS and FTD. Transcript levels of C9orf72 are reduced but probes for the hexanucleotide repeat identified intranuclear RNA foci within neurons in the frontal cortex implicating RNA mediated toxicity similar to myotonic dystrophy and some spinocerebellar ataxias [8].
Whilst the geneticists were closing in on the gene responsible on chromosome 9, Finnish pathologists described ubiquitin and p62 positive, TDP-43 negative, inclusions in the cerebellum, particularly in the granular layer, of their FTLD-TDP cases [26]. We have recently described characteristically shaped p62 positive cytoplasmic and intranuclear inclusions in the hippocampus and cerebellum in a proportion of cases of across the TDP-43 proteinopathy spectrum [13, 14]. These inclusions were also negative for tau, α synuclein, fused in sarcoma protein (FUS), α-internexin and neurofilament. Furthermore, most cases in our series appeared to be familial and had excess p62 pathology when compared to TDP-43. Here we sought to determine whether these p62 positive, TDP-43 negative inclusions correlate with the C9orf72 hexanucleotide repeat expansion in MND/ALS and FTLD.
Materials and methods
Identification of C9orf72 expansion using repeat primer PCR
DNA was extracted from frozen frontal lobe tissue in 36 cases of MND/ALS and FTLD and four normal controls. These were screened for the GGGGCC hexanucleotide repeat expansion, using the repeat primer PCR reaction, as previously described [8, 27]. Individual PCR reactions contained a final concentration of 7% DMSO, 1 M betaine, 0.17 mM of 7-deaza-2-dezoxy GTP, 0.7–1.4μM of primer mix, 0.85 mM of MgCl2, 50% Applied Biosystems True Allele PCR Premix and 100 ng of genomic DNA. The primer sequence mix consisted of a FAM-labelled reverse primer and two additional forward primers, one of which was repeat-specific and the other being an anchor [27]. Cycling conditions consisted of an initial denaturation step of 95°C for 15 min followed by touchdown from 70°C to 56°C with 3 min extension cycles. Fragment analysis of the PCR products was conducted on an ABI3130 DNA analyser and peaks visualized using Genemapper 4.0 software (Applied Biosystems, Warrington, United Kingdom).
Tissue collection and neuropathological assessment
Brain and spinal cord tissues were examined from 14 cases of MND/ALS and/or FTLD with a pathological C9orf72 expansion, 22 MND/ALS and FTLD cases without the expansion and four controls without the expansion. 10% formalin-fixed, paraffin-embedded tissue blocks were obtained from the Medical Research Council London Neurodegenerative Diseases Brain Bank (Institute of Psychiatry, King’s College London, UK). Consent for autopsy, neuropathological assessment and research was obtained from all subjects. Block taking for histological and immunohistochemical studies and neuropathological assessment for neurodegenerative diseases was performed in accordance with institutional guidelines. The clinicopathological diagnosis of MND/ALS and FTLD had been confirmed at post mortem histology by the presence of TDP-43 and phosphorylated-TDP-43 (p-TDP-43) positive neuronal cytoplasmic inclusions (NCIs) in the anterior horn cells of the spinal cord in MND/ALS and in the frontal and/or temporal neocortex in FTLD-TDP. For the purposes of this study the term FTLD-MND/ALS was only applied to cases where dementia was described clinically and there was also clinical evidence of MND/ALS, in addition to evidence pathologically of both MND/ALS and FTLD. Control cases had no cognitive decline or movement disorder (the only pathology being at most a modified tau Braak Alzheimer’s disease stage of II [1]). See Table 1 for full list and details of cases used.
Immunohistochemistry
Immunohistochemistry for p62 and phosphorylated TDP-43 was carried out on the frontal lobe containing the middle frontal gyrus, temporal lobe with superior and middle temporal gyrus, posterior hippocampus, cerebellum with dentate nucleus and spinal cord. The immunohistochemistry was conducted as per previously published protocols [21]. In brief, sections of 7 μm thickness were cut from the paraffin-embedded tissue blocks, deparaffinised in xylene, endogenous peroxidase was blocked by 2.5% H2O2 in methanol and immunohistochemistry performed. To enhance antigen retrieval sections were kept in citrate buffer for 10 min following microwave treatment. After blocking in normal serum (DAKO, Cambridgeshire, UK), primary antibody was applied overnight at 4°C (p62 mAb, BD Biosciences, 1:200 and p-TDP-43 (Ser409/410-2) pAb, Cosmobio, 1:4,500). Following washes, the sections were incubated with biotinylated secondary antibody (DAKO), followed by avidin:biotinylated enzyme complex (Vectastain Elite ABC kit, Vector Laboratories, Peterborough, UK). Finally, the sections were incubated for 10–15 min with 0.5 mg/mL 3,3′-diaminobenzidine chromogen (Sigma-Aldrich Company Ltd, Dorset UK) in Tris-buffered saline (pH 7.6) containing 0.05% H2O2. The sections were counterstained with Harris’ haematoxylin and immunostaining analysed by light microscopy.
C9orf72 antibodies were also tested on a number of cases with the expansion and a number without using the same protocol. Three commercially available antibodies were used, Sigma (HPA023873) Genetex (GTX119776) and SantaCruz (sc-138763) all at 1:50 and overnight incubation.
Double immunofluorescence was carried out with 7 μm sections cut from formalin-fixed paraffin-embedded blocks, de-waxed in xylene and dehydrated in 99% industrial methylated spirit. The sections were pre-treated by microwaving in citrate buffer and blocked using normal goat serum (1:10 for 45 min). Primary antibodies were then applied (phosphorylated TDP-43, 1:2,000, CosmoBio Ltd, Tokyo, Japan; p62, 1:200, BD Biosciences, Erembodegem, Belgium; FUS 1:200 Sigma, UK; Optineurin, 1:200 Proteintech, UK and Ubiquilin2, 1:200, Abnova, UK) and sections incubated at 37°C for 1 h. The sections were washed and secondary Alexa Fluor antibody (goat anti-mouse 568 and goat anti-rabbit 488, Invitrogen, Paisley, UK) applied for 45 min (in dark). Autofluorescence was quenched by incubating the sections in Sudan black for 10 min followed by numerous washes in phosphate buffered saline before coverslip mounting using hard set media with DAPI. The sections were visualised using a fluorescent microscope (Zeiss Axiovert S 100, Gottingen, Germany) and images captured using ImagePro Express (V6).
Results
Expansion mutation detection
Repeat primer PCR for the (GGGGCC)n hexanucleotide repeat within intron 1 of C9orf72 revealed a saw-tooth repeat fragment length pattern in 14 cases carrying the pathological expansion including: (a) nine MND/ALS, (b) four FTLD-TDP and (c) one FTLD-MND/ALS (Fig. 1).
The fragment analysis traces taken from Genemapper clearly show the presence of the expanded GGGGCC repeat from the repeat primed PCR reaction. The vertical axis represents fluorescent intensity with the horizontal axis displaying product size. An expansion positive case (a) manifests a saw-tooth pattern of individual peaks from 120 to 600 bp that indicate nucleotide repeats with a 6 bp interval in contrast to (b) an expansion negative sample
The mean age at death of MND/ALS cases with the repeat expansion was 55 years compared to 61 years without the expansion and in FTLD cases the mean age at death was 61 years with the repeat expansion and 66 years without the repeat expansion. In MND/ALS cases with the repeat expansion the mean brain weight was 1,362 g (range 1,269–1,563 g) compared to a mean brain weight of 1,402 g in those MND/ALS cases without the repeat expansion (range 1,164–1,594 g). The mean brain weight of FTLD cases with the repeat expansion was 1,078 g (range 999–1,215 g) compared to 1,022 g in those FTLD cases without the expansion (range 796–1,182 g). The sample size was small, but statistical tests revealed no significant difference between age at death or brain weight between MND/ALS cases with and without the repeat expansion or FTLD cases with and without the repeat expansion.
Immunohistochemistry
A summary comparing the histological and immunohistochemical features of cases with and without the hexanucleotide repeat expansion is shown in Table 2. In all the 14 expansion positive cases numerous p62 positive neuronal cytoplasmic inclusions (NCIs) were seen in the cerebellum. This was the most evident in the granular layer where there were numerous NCIs (Fig. 2a). p62 positive, p-TDP-43 negative, NCIs were detected in the molecular layer in all cases and within Purkinje cells in 12/14 cases (Fig. 2a, b). In only one case (case 1) were p-TDP-43 inclusions detected in the granular cell layer of the cerebellum (Fig. 2c). p62 positive, p-TDP-43 negative neuronal intranuclear inclusions (NIIs) were also detected within the granular layer in 6/14 cases (Fig. 2d–g) and in a Purkinje cell of one case (Fig. 2d) but there was no obvious neuronal depletion in any of the cases.
Cerebellum: histological sections of cerebellum from cases with the C9orf72 repeat expansion. a A p62 immunoreactive NCI in the Purkinje cell (arrow) and in many granular cells (case 1) (scale bar 10 μm). b Many p62 positive NCIs in the cells of the molecular layer (case 13) (scale bar 10 μm). c One case only (case 1) showed p-TDP-43 positive NCIs (scale bar 30 μm) in the granular cell layer. d p62 positive NII in Purkinje cell (white arrow) and granule cell (black arrow) (case 7) (scale bar 10 μm). e–g p62 positive NIIs in granular cells of the cerebellum (black arrows) (cases 5, 13 and 13) (scale bar 10 μm)
p62 positive inclusions were also prominent within the hippocampus where in most cases they appeared to be more abundant than p-TDP-43 positive inclusions (Fig. 3a, b). In all the repeat expansion positive cases (14/14) numerous large irregular and star-shaped p62 positive NCIs were observed, particularly in the CA4 region (endfolium) (Fig. 3c–e). Only a very small proportion of p62 positive inclusions were also immunoreactive for p-TDP-43 and these were seen in 8/14 cases. p62 positive, p-TDP-43 negative NIIs were also abundant within the pyramidal cell layer of the hippocampus, being readily observed in 12/14 cases (Fig. 3e–g). They ranged in size from dot-like structures (Fig. 3e, g) to spheres approximating the size of the nucleolus (Fig. 3f). Again no significant neuronal loss was evident. There was abundant p62 positivity in the dentate gyrus in the form of NCIs in all cases (Fig. 3h). p-TDP-43 positive inclusions were seen in this layer in 13/14 cases, but in the majority of cases they were less frequent than those labelled by p62 (Fig. 3i).
Hippocampus: histological sections from the hippocampus of cases with the C9orf72 repeat expansion and stained with p62 and p-TDP-43. a CA4 (endfolium) with frequent p62 positive NCIs. (case 12) b Same region as (a) but stained with p-TDP-43 and showing no recognizable immunostaining in the cytoplasm of pyramidal cells. (case 12) (Scale bar 100 μm). c, d Typical p62 immunopositive irregular globules or star-shaped NCIs in the neurons of the CA4 region (cases 8 and 4) (scale bar 30 μm). e–g p62 positive NIIs in the neurons of the CA4 region (cases 9, 6 and 8) (scale bar 20 μm). h Frequent p62 positive NCIs in the dentate fascia (case 11) (scale bar 25 μm). i Same region as (h) with less frequent p-TDP-43 immunostained NCIs (case 11) (scale bar 50 μm)
Within the neocortex two distinct patterns of p62 and p-TDP-43 inclusion distribution were observed. In the upper layers of the frontal and temporal cortex in the FTLD-TDP and FTLD-MND/ALS cases filamentous, semi-lunar and dot-like NCIs positive for both p-TDP-43 and p62 were observed. This explained the otherwise comparable densities of p-TDP-43 and p62 in the cortex in Table 2. However, there were also distinct irregular and star-shaped p62 positive NCIs most frequently identified in the inner granular and lower polymorphic layers (Fig. 4a, c, e and f). Only a small proportion of these latter NCIs were also immunoreactive for p-TDP-43, appearing as irregular or granular inclusions (Fig. 4b, d). Indeed this was the pattern which tended to be seen in the MND/ALS cases and overall p-TDP43 inclusions were completely absent in the temporal lobe in 5/14 cases and frontal lobe in 4/14 cases.
Cerebral cortex: histological sections from temporal lobe from cases with the C9orf72 repeat expansion and stained with p62 and p-TDP-43. a, c p62 immunostaining from the lower layers of the temporal cortex showing frequent p62 globular and star-shaped NCI in the small neurons (cases 13 and 10) (scale bar 25 μm). b, d Same stained area with p-TDP43 showing less p-TDP-43 staining in the NCIs as small globules or diffuse granular staining (cases 13 and 10) (scale bar 25 μm). e, f Higher power of neurons with globular and star shaped p62 positive NCIs in small and moderate size neurons (case 12) (scale bar 10 μm)
p62 positive NIIs were also seen in the temporal cortical neurons in 5/14 cases but these were less frequent than the NCIs.
In the hippocampus very occasional p62 positive dystrophic neurites were seen in 9/14 cases (not shown), and of these only five had occasional additional positivity for p-TDP-43.
Within the neocortex, neurites (p62 and p-TDP-43 positive) were seen only occasionally in three MND/ALS cases (not shown) and were completely absent in five MND/ALS cases and moderately frequent in one MND/ALS case. Of the remaining five FTLD-TDP and FTLD-MND/ALS cases, p62 positive, p-TDP-43 positive neurites were abundant in two cases and moderately frequent in three cases (mainly in the upper cortical layers).
Spinal cord sections were available from eight MND/ALS cases with the repeat expansion. Typical skein-like and globular inclusions were positive for both p62 and p-TDP-43 and staining appeared to be equally abundant.
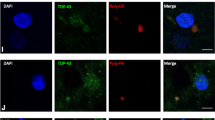
We tested a range of optimising conditions for the three commercially available antibodies reputed to recognise the C9orf72 protein (GeneTex, Santa Cruz and Sigma) and only the Sigma antibody showed reactivity in paraffin-embedded tissues, although this appeared to be fairly non-specific (not shown). Double labelling immunofluorescence failed to reveal any co-localisation of p62 and the C9orf72 protein, as visualised using this antibody. Nor did the p62 positive inclusions co-localise with other known MND/ALS and FTLD associated proteins including fused in sarcoma and optineurin. Double labelling also confirmed the immunohistochemical observation that numerous p62 inclusions were not also p-TDP-43 positive (Fig. 5).
Double immunoflourescence staining in the hippocampal region of a C9orf72repeat expansion positive case (case 9). a p62 (green) inclusions do not co-localise with C9orf72 (red). (scale bar 10 μm) b Only a minority of p62 (green) inclusions are also positive for p-TDP-43 (red) (arrows show p62 only positive inclusions, arrowhead shows one inclusion labelled with both p62 and p-TDP-43) (scale bar 20 μm)
Tissues from 22 cases without the repeat expansion, 15 diagnosed with MND/ALS and 7 with FTLD-TDP, also underwent detailed immunohistochemistry (see Table 2). In FTLD cases characteristic p62 and p-TDP43 positive filamentous, dot-like and semi-lunar shaped NCIs were detected in the upper layers of the cerebral cortex in the frontal and temporal lobes, and the dentate fascia of the hippocampus. No p62 positive p-TDP-43 negative, star-like NCIs were detected in any case without the expansion. Occasional p62 and p-TDP43 positive NIIs were observed in the cerebral cortex but not in the endfolium (CA4). No p62 positive, p-TDP-43 negative inclusions were seen in the cerebellum. Three of the 15 MND/ALS cases had p-TDP-43 and p62 positive NCIs in the upper layers of the cortex and 4/15 had p-TDP-43 and p62 positive NCIs in the small neurons of the dentate fascia. None of these cases were identified in life as having cognitive deficits.
The spinal cord in all the repeat expansion negative MND/ALS cases showed typical p62 and p-TDP-43 positive globular and skein like inclusions in neurons and glia without an excess of p62 positivity and no NIIs were seen.
Discussion
The repeat expansion in intron 1 of C9orf72 is reported to be the most common gene mutation in familial and sporadic MND/ALS and FTLD [8, 27]. We, and others, have previously described p62 positive cerebellar inclusions in MND/ALS and FTLD-TDP [13, 14, 26]. These inclusions have so far been immuno-negative for non- phosphorylated TDP-43, but our previous study did reveal p-TDP-43 positive cerebellar inclusions in one case [14]. In one study these cerebellar inclusions were linked to chromosome 9p [5]. Our results demonstrate immunohistological features that are pathognomonic for those FTLD and MND/ALS cases carrying the C9orf72 repeat expansion:
-
1.
p62 positive and p-TDP-43 negative NCIs in the granular cell layer, molecular layer and Purkinje cells of the cerebellum and pyramidal cell layer of the hippocampus
-
2.
p62 positive and p-TDP-43 negative spherical NIIs in the pyramidal cell layer of hippocampus and the granular cell layer of the cerebellum.
The pathology was so distinct that cases with the C9orf72 repeat expansion could be identified blindly to genotype by examining p62 immunohistochemistry of the hippocampus and cerebellum. There were occasional NIIs also identified in the cerebral cortex in some cases and in a Purkinje cell in one case, but these were not frequent enough to be relied upon as distinctive features.
The findings in the cerebral cortex were particularly interesting, yet complex. In the hexanucleotide repeat expansion FTLD-TDP cases there was positivity for p62 and p-TDP-43 but only in the superficial cortex did these NCIs tend to be positive for both antibodies. Deeper in the cortex the p62 positive, p-TDP-43 negative inclusions tended to predominate. The hexanucleotide repeat expansion MND/ALS cases tended to show p62 positive inclusions in the deeper cortical layers with little corresponding positivity for p-TDP-43. It is recognised that MND/ALS cases can exhibit TDP-43 positive extramotor inclusions in the hippocampus and occasionally in the neocortex without necessarily being re-labelled as FTLD-MND/ALS [10] (especially when this is defined with rigid clinical and pathological criteria as in this study), but the predominace of p62 NCIs in many cases is very unusual.
p62, or sequestosome 1 as it is sometimes known, is a ubiquitin binding protein that facilitates the degradation of polyubiquitinated proteins by the ubiquitin-proteosome system (UPS) or by autophagosome–lysosome pathways [34]. Typical MND/ALS and FTLD-TDP is characterised by NCIs that are positive for ubiquitin, p-TDP-43 and p62. Neurodegeneration in chromosome 9p linked MND/ALS and FTLD cases is assumed to be due to TDP-43 mislocalisation and aggregation, which may well be the case, but the frequent and widespread appearance of p62 positive inclusions that were negative for p-TDP-43 suggests that other proteins accumulating in the cytoplasm and nucleus may contribute to cell death. We have demonstrated that these inclusions do not contain the C9orf72 protein itself nor any other known MND/ALS FTLD associated proteins such as fused in sarcoma (FUS), and optineurin. Furthermore, despite the abundance of inclusions in the cerebellum there was no conspicuous evidence of neuronal loss.
Probes for the GGGGCC repeat detected foci of RNA accumulating within the nuclei of ~25% of neurons in the frontal cortex of a patient with the expansion mutation [8]. Our frequent observation of dot-like p62 positive NIIs within pyramidal neurons in the hippocampus and granular cells in the cerebellum indicates that proteins are also accumulating in the nucleus, possibly in association with RNA foci. Interestingly, polyubiquitinated and p62 positive intranuclear inclusions are a feature of other intronic expansion mutation diseases such as myotonic dystrophy (DM 1 and 2) and several spinocerebellar ataxias (SCA 8, 10, 32, 35, FXTAS) (reviewed in [31]). Ubiquitinated and p62 inclusions also occur in many polyglutamine tract diseases so their appearance is not disease-specific [3]. The dominant intronic nucleotide repeat disorders are thought to cause toxicity and dysfunction by affecting RNA transcription and splicing in part through the sequestration of RNA binding proteins. For DM1 the CTG repeat is sufficient to induce toxicity in mice and Drosophila due in part to the sequestration of several members of the muscleblind-like (MBNL) family of RNA splicing proteins which results in aberrant splicing occurring in several key genes [31].
The pathological features we have described in our C9orf72-associated FTLD cases are difficult to ascribe to a single pathological subtype according to existing criteria [6, 20, 28]. We therefore support a new pathological subtype of C9orf72-ALS/FTLD as defined by characteristic p62 positive, p-TDP-43 negative cytoplasmic and nuclear inclusions. The identity of the polyubiquitinated proteins bound to p62 in the distinctive inclusions and their role, if any, in pathogenesis remains to be established and is the focus of future research.
References
Alafuzoff I, Arzberger T, Al-Sarraj S et al (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18:484–496
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Becher MW, Kotzuk JA, Sharp AH et al (1998) Intranuclear neuronal inclusions in Huntington’s disease and dentatorubral and pallidoluysian atrophy: correlation between the density of inclusions and IT15 CAG triplet repeat length. Neurobiol Dis 4:387–397
Beck J, Rohrer JD, Campbell T et al (2008) A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 131:706–720
Boxer AL, Mackenzie IR, Boeve BF et al (2010) Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry 82:196–203
Cairns NJ, Bigio EH, Mackenzie IR et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol 114:5–22
Cairns NJ, Neumann M, Bigio EH et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Dejesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 72:245–256
Deng HX, Chen W, Hong ST et al (2011) Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477:211–215
Geser F, Brandmeir NJ, Kwong LK et al (2008) Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol 65:636–641
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Johnson JO, Mandrioli J, Benatar M et al (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68:857–864
King A, Al-Sarraj S, Shaw C (2009) Frontotemporal lobar degeneration with ubiquitinated tau-negative inclusions and additional alpha-synuclein pathology but also unusual cerebellar ubiquitinated p62-positive, TDP-43-negative inclusions. Neuropathology 29:466–471
King A, Maekawa S, Bodi I et al (2011) Ubiquitinated, p62 immunopositive cerebellar cortical neuronal inclusions are evident across the spectrum of TDP-43 proteinopathies but are only rarely additionally immunopositive for phosphorylation-dependent TDP-43. Neuropathology 31:239–249
Laaksovirta H, Peuralinna T, Schymick JC et al (2010) Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol 9:978–985
Leigh PN, Anderton BH, Dodson A et al (1988) Ubiquitin deposits in anterior horn cells in motor neurone disease. Neurosci Lett 93:197–203
Lowe J, Lennox G, Jefferson D et al (1988) A filamentous inclusion body within anterior horn neurones in motor neurone disease defined by immunocytochemical localisation of ubiquitin. Neurosci Lett 94:203–210
Mackenzie IR (2007) The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 114:49–54
Mackenzie IR, Baker M, Pickering-Brown S et al (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Mackenzie IR, Neumann M, Bigio EH et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Maekawa S, Leigh PN, King A et al (2009) TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology 29:672–683
Maruyama H, Morino H, Ito H et al (2010) Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465:223–226
Morita M, Al-Chalabi A, Andersen PM et al (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66:839–844
Neumann M, Mackenzie IR, Cairns NJ et al (2007) TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol 66:152–157
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Pikkarainen M, Hartikainen P, Alafuzoff I (2008) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions visualized with ubiquitin-binding protein p62 immunohistochemistry. J Neuropathol Exp Neurol 67:280–298
Renton AE, Majounie E, Waite A et al (2011) A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron 72:257–268
Sampathu DM, Neumann M, Kwong LK et al (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Shatunov A, Mok K, Newhouse S et al (2010) Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol 9:986–994
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Todd PK, Paulson HL (2010) RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol 67:291–300
Van Deerlin VM, Sleiman PM, Martinez-Lage M et al (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42:234–239
Vance C, Al-Chalabi A, Ruddy D et al (2006) Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain 129:868–876
Wooten MW, Hu X, Babu JR et al (2006) Signaling, polyubiquitination, trafficking, and inclusions: sequestosome 1/p62’s role in neurodegenerative disease. J Biomed Biotechnol 2006:62079
Acknowledgments
The authors thank the donors and their families whose donation of brain and spinal cord tissue to the Medical Research Council London Neurodegenerative Diseases Brain Bank allowed this research to take place. The authors thank Professor Martin Rossor for referral of GRN mutation cases. They also thank the staff of the Clinical Neuropathology Department, King’s College Hospital, especially Mary Davitt and Joanne Hickey. Funding to support this work came from the Motor Neuron Disease Association, American Amyotrophic Lateral Sclerosis Association, Heaton-Ellis Trust, Medical Research Council (UK), Wellcome Trust and Psychiatry Research Trust.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors S. Al-Sarraj and A. King have made an equal contribution.
Rights and permissions
About this article
Cite this article
Al-Sarraj, S., King, A., Troakes, C. et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122, 691–702 (2011). https://doi.org/10.1007/s00401-011-0911-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0911-2