Abstract
Rationale
Reversal learning deficits are a feature of many human psychopathologies and their associated animal models and have recently been shown to involve the 5-HT2C receptor (5-HT2CR). Successful reversal learning can be reduced to two dissociable cognitive mechanisms, to dissipate associations of previous positive (opposed by perseverance) and negative (opposed by learned non-reward) valence.
Objectives
This study aims to explore the effect of reducing activity at the 5-HT2CR on the cognitive mechanisms underlying spatial reversal learning in the mouse.
Methods
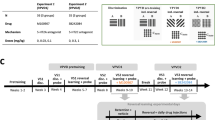
Experiment 1 used the 5-HT2CR antagonist SB242084 (0.5 mg/kg) in a between-groups serial design, experiment 2 used 5-HT2CR KO mice in a repeated measures design. Animals initially learned to discriminate between two lit nosepoke holes. This was followed by three conditions; (1) full reversal, where contingencies reversed; (2) perseverance, where the previous CS+ became CS− and the previous CS− was replaced by a novel CS+; (3) learned non-reward, where the previous CS− became CS+ and the previous CS+ was replaced by a novel CS−.
Results
SB242084 treated and 5-HT2CR KO mice showed enhanced reversal learning seen as a decrease in trials, correct responses, and omissions to criterion in the full reversal condition. Similar effects were observed in the learned non-reward condition but SB242084 treated and 5-HT2CR KO mice did not differ from controls in the perseverance condition. SB242084 treated, but not 5-HT2CR KO mice, showed decreases in all latency indices in every condition.
Conclusion
Reducing activity at the 5-HT2CR facilitates reversal learning in the mouse by reducing the influence of previously non-rewarded associations.





Similar content being viewed by others

References
Abdallah L, Bonasera SJ, Hopf FW et al (2009) Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci 29:8156–8165. doi:10.1523/jneurosci.3905-08.2009
Asin KE, Wirtshafter D, Kent EW (1980) The effects of electrolytic median raphe lesions on two measures of latent inhibition. Behav Neural Biol 28:408–417. doi:10.1016/S0163-1047(80)91734-3
Baxter MG, Holland PC, Gallagher M (1997) Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J Neurosci 17:5230–5236
Beran MJ, Klein ED, Evans TA et al (2008) Discrimination reversal learning in capuchin monkeys (Cebus apella). Psychol Rec 58:3–14
Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM (2008) Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci 28:11124–11130. doi:10.1523/JNEUROSCI.2820-08.2008
Bolla KI, Eldreth DA, London ED et al (2003) Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage 19:1085–1094. doi:10.1016/s1053-8119(03)00113-7
Bonsi P, Cuomo D, Ding J et al (2007) Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32:1840–1854. doi:10.1038/sj.npp.1301294
Boothman LJ, Allers KA, Rasmussen K, Sharp T (2003) Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol 139:998–1004. doi:10.1038/sj.bjp.0705328
Boothman LJ, Raley J, Denk F (2006) In vivo evidence that 5-HT2C receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Brit J Pharmacol 149:861–869. doi:10.1038/sj.bjp.0706935
Boulougouris V, Robbins TW (2010) Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci 30:930–938. doi:10.1523/JNEUROSCI.4312-09.2010
Boulougouris V, Dalley JW, Robbins TW (2007) Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res 179:219–228. doi:10.1016/j.bbr.2007.02.005
Boulougouris V, Glennon JC, Robbins TW (2008) Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacol 33:2007–2019. doi:10.1038/sj.npp.1301584
Boulougouris V, Castañé A, Robbins TW (2009) Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology 202:611–620. doi:10.1007/s00213-008-1341-2
Brigman JL, Mathur P, Harvey-White J et al (2010) Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex 20:1955–1963. doi:10.1093/cercor/bhp266
Bubar MJ, Cunningham KA (2007) Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146:286–297. doi:10.1016/j.neuroscience.2006.12.071
Burke KA, Takahashi YK, Correll J, Takahashi YK, Correll J, Brown PL, Schoenbaum G (2009) Orbitofrontal inactivation impairs reversal of Pavlovian learning by interfering with “disinhibition” of responding for previously unrewarded cues. Eur J Neurosci 30:1941–1946. doi:10.1111/j.1460-9568.2009.06992.x
Cassaday H, Hodges H, Gray J (1993a) The effects of ritanserin, RU 24969 and 8-OH-DPAT on latent inhibition in the rat. J Psychopharmacol 63–71. doi:10.1177/026988119300700110
Cassaday HJ, Mitchell SN, Williams JH, Gray JA (1993b) 5,7-Dihydroxytryptamine lesions in the fornix-fimbria attenuate latent inhibition. Behav Neural Biol 59:194–207. doi:10.1016/0163-1047(93)90962-H
Ceaser AE, Goldberg TE, Egan MF, McMahon RP, Weinberger DR, Gold JM (2008) Set-shifting ability and schizophrenia: a marker of clinical illness or an intermediate phenotype? Biol Psychiatry 64:782–788. doi:10.1016/j.biopsych.2008.05.009
Chamberlain SR, Müller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863. doi:10.1126/science.1121218
Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC (2004) Cognitive inflexibility after prefrontal serotonin depletion. Science 304:878–880. doi:10.1126/science.1094987
Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC (2005) Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci 25:532–538. doi:10.1523/JNEUROSCI.3690-04.2005
Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC (2007) Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17:18–27. doi:10.1093/cercor/bhj120
D'Amato MR, Jagoda H (1961) Analysis of the role of overlearning in discrimination reversal. J Exp Psychol 61:45–50. doi:10.1037/h0047757
Dalton GL, Lee MD, Kennett GA, Dourish CT, Clifton PG (2006) Serotonin 1B and 2C receptor interactions in the modulation of feeding behaviour in the mouse. Psychopharm 185:45–57. doi:10.1007/s00213-005-0212-3
Danet M, Lapiz-Bluhm S, Soto-Pina AE, Hensler JG, Moralik DA (2009) Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharm 202:329–341. doi:10.1007/s00213-008-1224-6
Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E (1999) SB 242 084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharm 38:1195–1205
Dias R, Robbins TW, Roberts AC (1996) Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380:69–72. doi:10.1038/380069a0
Drevets WC, Videen TO, Price JL et al (1992) A functional anatomical study of unipolar depression. J Neurosci 12:3628–3641
Dunn L, Scibilia R, Franks J, Kilts C (1991) Comparison of the effect on latent inhibition of dopamine agonists and atypical antipsychotics in rats. Abstr Soc Neurosci 17:99
Elliott R, McKenna PJ, Robbins TW, Sahakian BJ (1995) Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med 25:619–630
Elliott R, McKenna PJ, Robbins TW (1998) Specific neuropsychological deficits in schizophrenic patients with preserved intellectual function. Cogn Neuropsychiatry 3:45–70. doi:10.1080/135468098396242
Ersche KD, Roiser JP, Robbins TW, Sahakian BJ (2008) Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology 197:421–431. doi:10.1007/s00213-007-1051-1
Fletcher PJ (1993) A comparison of the effects of dorsal or median raphe injections of 8-OH-DPAT in three operant tasks measuring response inhibition. Behav Brain Res 54:187–197. doi:10.1016/0166-4328(93)90078-5
Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology 195:223–234. doi:10.1007/s00213-007-0891-z
Fletcher PJ, Tampakeras M, Sinyard J et al (2009) Characterizing the effects of 5-HT2C receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology 57:259–267. doi:10.1016/j.neuropharm.2009.05.011
Fletcher PJ, Sinyard J, Higgins GA (2010) Genetic and pharmacological evidence that 5-HT2C receptor activation, but not inhibition, affects motivation to feed under a progressive ratio schedule of reinforcement. PBB 97:170–178. doi:10.1016/j.pbb.2010.07.002
Freedman M, Oscar-Berman M (1989) Spatial and visual learning deficits in Alzheimer's and Parkinson's disease. Brain Cogn 11:114–126. doi:10.1016/0278-2626(89)90009-2
Gobert A, Rivet JM, Lejeune F et al (2000) Serotonin2C receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse 36:205–221. doi:10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D
Goulart PRK, Mendonça MB, Barros RS, Galvao OF, McIlvane WJ (2005) A note on select- and reject-controlling relations in the simple discrimination of capuchin monkeys (Cebus apella). Behav Processes 69:295–302. doi:10.1016/j.beproc.2004.12.005
Green MF (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67(Suppl 9):3–8
Green MF, Braff DL (2001) Translating the basic and clinical cognitive neuroscience of schizophrenia to drug development and clinical trials of antipsychotic medications. Biol Psychiatry 49:374–384. doi:10.1016/S0006-3223(00)01027-1
Haluk DM, Floresco SB (2009) Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology 34:2041–2052. doi:10.1038/npp.2009.21
Harlow HF, Hicks LH (1957) Discrimination learning theory: uniprocess vs. duoprocess. Psychol Rev 64:104–109
Hewitt KN, Lee MD, Dourish CT, Clifton PG (2002) Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. PBB 71:691–700. doi:10.1016/S0091-3057(01)00709-2
Higgins GA, Fletcher PJ (2003) Serotonin and drug reward: focus on 5-HT2C receptors. Eur J Pharmacol 480:151–162. doi:10.1016/j.ejphar.2003.08.102
Higgins GA, Enderlin M, Haman M, Fletcher PJ (2003) The 5-HT2A receptor antagonist M100,907 attenuates motor and “impulsive-type” behaviours produced by NMDA receptor antagonism. Psychopharmacology 170:309–319. doi:10.1007/s00213-003-1549-0
Hsu JL, Yu L, Sullivan E, Bowman M, Mistlberger RE, Tecott LH (2010) Enhanced food anticipatory activity associated with enhanced activation of extrahypothalamic neural pathways in serotonin2C receptor null mutant mice. PLoS One 5:e11802. doi:10.1371/journal.pone.0011802
Hudson AJ (1968) Perseveration. Brain 91:571–582. doi:10.1093/brain/91.3.571
Idris NF, Repeto P, Neill JC, Large CH (2005) Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and d-amphetamine in the rat. Psychopharmacology 179:336–348. doi:10.1007/s00213-004-2058-5
Kaplan O, Dar R, Rosenthal L, Hermesh H, Fux M, Lubow RE (2006) Obsessive-compulsive disorder patients display enhanced latent inhibition on a visual search task. Behav Res Ther 44:1137–1145. doi:10.1016/j.brat.2005.09.005
Kennett GA, Wood MD, Bright F et al (1997) SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36:609–620. doi:10.1016/S0028-3908(97)00038-5
Kim J, Ragozzino ME (2005) The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem 83:125–133. doi:10.1016/j.nlm.2004.10.003
Lacerda ALT, Dalgalarrondo P, Caetano D, Haas GL, Camargo EE, Keshavan MS (2003) Neuropsychological performance and regional cerebral blood flow in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 27:657–665. doi:10.1016/S0278-5846(03)00076-9
Lawrence AD, Sahakian BJ, Rogers RD, Hodges JR, Robbins TW (1999) Discrimination, reversal, and shift learning in Huntington's disease: mechanisms of impaired response selection. Neuropsychologia 37:1359–1374. doi:10.1016/S0028-3932(99)00035-4
Lee B, Groman S, London ED, Jentsch JD (2007) Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology 32:2125–2134. doi:10.1038/sj.npp.1301337
Leeson VC, Robbins TW, Matheson E et al (2009) Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 66:586–593. doi:10.1016/j.biopsych.2009.05.016
Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA (2007) Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience 146:1677–1688. doi:10.1016/j.neuroscience.2007.02.064
Lorden JF, Rickert EJ, Berry DW (1983) Forebrain monoamines and associative learning: I. Latent inhibition and conditioned inhibition. Behav Brain Res 9:181–199. doi:10.1016/0166-4328(83)90127-4
Loskutova LV (2001) The effects of a serotoninergic substrate of the nucleus accumbens on latent inhibition. Neurosci Behav Physiol 31:15–20. doi:10.1023/A:1026613928155
Loskutova LV, Luk'yanenko FY, Il'yuchenok RY (1990) Modeling of latent inhibition in rats by activation of the central serotoninergic system. Neurosci Behav Physiol 20:541–542. doi:10.1007/BF01237281
Mackintosh NJ (1983) Conditioning and associative learning. Oxford University Press, New York
Maes JHR, Eling PATM (2002) Do learned irrelevance and perseveration play a role during discrimination learning? Learn Motiv 40:274–283. doi:10.1016/j.lmot.2009.02.001
Maes JHR, Damen MDC, Eling PATM (2004) More learned irrelevance than perseveration errors in rule shifting in healthy subjects. Brain Cogn 54:201–211. doi:10.1016/j.bandc.2004.01.003
Masaki D, Yokoyama C, Kinoshita S et al (2006) Relationship between limbic and cortical 5-HT neurotransmission and acquisition and reversal learning in a go/no-go task in rats. Psychopharmacology 189:249–258. doi:10.1007/s00213-006-0559-0
Mason JR, Stevens DA, Wixon DR, Owens MP (1980) Assessment of the relative importance of S+ and S− in rats using differential training on intercurrent discriminations. Learn Motiv 11:49–60. doi:10.1016/0023-9690(80)90020-X
McAlonan K, Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146:97–103. doi:10.1016/j.bbr.2003.09.019
McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, McIntosh AM (2009) Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med 39:1289–1293. doi:10.1017/S0033291708004935
McLean SL, Woolley ML, Thomas D, Neill JC (2009) Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology 206:403–414. doi:10.1007/s00213-009-1618-0
Molodtsova GF (2003) Differences in serotonin and dopamine metabolism in the rat brain in latent inhibition. Neurosci Behav Physiol 33:217–222
Molodtsova GF (2008) Serotonergic mechanisms of memory trace retrieval. Behav Brain Res 195:7–16. doi:10.1016/j.bbr.2007.12.005
Morrison SE, Saez A, Lau B, Salzman CD (2011) Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron 71:1127–1140. doi:10.1016/j.neuron.2011.07.016
Moss EM, Harlow HF (1947) The role of reward in discrimination learning in monkeys. J Comp Physiol Psych 40:333–342. doi:10.1037/h0058469
Mullins GP, Winefield AH (1979) The relative importance of responses to S+ and S− in simultaneous discrimination learning. Q J Exp Psychol 31:329–338. doi:10.1080/14640747908400731
Nair SG, Gudelsky GA (2004) Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse 53:202–207. doi:10.1002/syn.20054
Nonkes LJP, Tomson K, Mærtin A, Dederen J, Maes JHR, Homberg J (2010) Orbitofrontal cortex and amygdalar over-activity is associated with an inability to use the value of expected outcomes to guide behaviour in serotonin transporter knockout rats. Neurobiol Learn Mem 94:65–72. doi:10.1016/j.nlm.2010.04.002
Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH (2003) Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes 52:315–320. doi:10.2337/diabetes.52.2.315
Owen AM, Roberts AC, Polkey CE, Sahakian BJ (1991) Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 29:993–1006. doi:10.1016/0028-3932(91)90063-E
Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW (1993) Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain 1159–1175. doi:10.1093/brain/116.5.1159
Pompeiano M, Palacios JM, Mengod G (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 23:163–178. doi:10.1016/0169-328X(94)90223-2
Pozzi L, Invernizzi R, Garavaglia C (1999) Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin. J Neurochem 73:1051–1057. doi:10.1046/j.1471-4159.1999.0731051.x
Quérée P, Peters S, Sharp T (2009) Further pharmacological characterization of 5-HT(2C) receptor agonist-induced inhibition of 5-HT neuronal activity in the dorsal raphe nucleus in vivo. Br J Pharmacol 158:1477–1485. doi:10.1111/j.1476-5381.2009.00406.x
Roberts AC (2011) The importance of serotonin for orbitofrontal function. Biol Psychiatry 69:1185–1191. doi:10.1016/j.biopsych.2010.12.037
Rocha BA, Goulding EH, O'Dell LE et al (2002) Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci 22:10039–10045
Rolls ET (2000) The orbitofrontal cortex and reward. Cereb Cortex 10:284–294. doi:10.1093/cercor/10.3.284
Rolls ET, Hornak J, Wade D et al (1994) Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatr 57:1518–1524
Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E (2009) Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharm 57:640–652. doi:10.1016/j.neuropharm.2009.08.013
Rueter LE, Tecott LH, Blier P (2000) In vivo electrophysiological examination of 5-HT2 responses in 5-HT2C receptor mutant mice. N-S Arch Pharmacol 361:484–491. doi:10.1007/s002109900181
Saxena S, Brody AL, Maidment KM et al (1999) Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharm 21:683–693. doi:10.1016/S0893-133X(99)0082-2
Schiller D, Weiner I (2004) Lesions to the basolateral amygdala and the orbitofrontal cortex but not to the medial prefrontal cortex produce an abnormally persistent latent inhibition in rats. Neuroscience 128:15–25. doi:10.1016/j.neuroscience.2004.06.020
Schiller D, Zuckerman L, Weiner I (2006) Abnormally persistent latent inhibition induced by lesions to the nucleus accumbens core, basolateral amygdala and orbitofrontal cortex is reversed by clozapine but not by haloperidol. J Psychiatr Res 40:167–177. doi:10.1016/j.jpsychires.2005.03.002
Shadach E, Gaisler I, Schiller D, Weiner I (2000) The latent inhibition model dissociates between clozapine, haloperidol, and ritanserin. Neuropsychopharmacology 23:151–161. doi:10.1016/S0893-133X(00)00096-8
Simpson EH, Kellendonk C, Ward RD et al (2011) Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry 69:928–935. doi:10.1016/j.biopsych.2011.01.012
Slabosz A, Lewis SJG, Smigasiewicz K, Szymura B, Barker RA, Owen AM (2006) The role of learned irrelevance in attentional set-shifting impairments in Parkinson's disease. Neuropsychology 20:578–588. doi:10.1037/0894-4105.20.5.578
Solomon PR, Kiney CA, Scott DR (1978) Disruption of latent inhibition following systemic administration of parachlorophenylalanine (PCPA). Physiol Behav 20:265–271. doi:10.1016/0031-9384(78)90219-6
Solomon PR, Nichols GL, Kiernan JM, Kamer RS, Kaplan LJ (1980) Differential effects of lesions in medial and dorsal raphe of the rat: latent inhibition and septohippocampal serotonin levels. J Comp Physiol Psychol 94:145–154. doi:10.1037/h0077655
Spence KW (1936) The nature of discrimination learning in animals. Psychol Rev 43:427–449. doi:10.1037/h0056975
Sutherland NS, Mackintosh NJ (1971) Mechanisms of animal discrimination learning. Academic, London
Swedo SE, Schapiro MG, Grady CL et al (1989) Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry 46:518–523
Swerdlow NR, Hartston HJ, Hartman PL (1999) Enhanced visual latent inhibition in obsessive-compulsive disorder. Biol Psychiat 45:482–488
Tait DS, Brown VJ (2007) Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci 1121:407–420. doi:10.1196/annals.1401.010
Tecott LH, Sun LM, Akana SF et al (1995) Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 374:542–546. doi:10.1038/374542a0
Tsaltas E, Boulougouris V (2011) The role of serotonin on attentional processes and executive functioning: Focus on 5-HT2C receptors. In: Di Giovanni G, Esposito E, Di Matteo V (eds) 5-HT2C receptors in the pathophysiology of CNS disease. The Receptors, vol. 22. Humana Press, New York, pp 445–460. doi:10.1007/978-1-60761-941-3
Valerius G, Lumpp A, Kuelz AK, Freyer T, Voderholzer U (2008) Reversal learning as a neuropsychological indicator for the neuropathology of obsessive compulsive disorder? A behavioral study. J Neuropsychiatry Clin Neurosci 20:210–218. doi:10.1176/appi.neuropsych.20.2.210
Weiner I, Arad M (2009) Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res 204:369–386. doi:10.1016/j.bbr.2009.05.004
Winstanley CA, Theobald DEH, Dalley JW, Glennon JC, Robbins TW (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology 176:376–385. doi:10.1007/s00213-004-1884-9
Zhelyazkova-Savova M, Giovannini MG, Pepeu G (1997) Increase of cortical acetylcholine release after systemic administration of chlorophenylpiperazine in the rat: an in vivo microdialysis study. Neurosci Lett 236:151–154. doi:10.1016/S0304-3940(97)00785-4
Zhelyazkova-Savova M, Giovannini MG, Pepeu G (1999) Systemic chlorophenylpiperazine increases acetylcholine release from rat hippocampus-implication of 5-HT2C receptors. Pharmacol Res 40:165–170. doi:10.1006/phrs.1999.0473
Acknowledgements
Supported by BBSRC and Eli Lilly through CASE studentship (BB/F529054/1). The authors would like to thank Lindsey Welstead for genotyping.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nilsson, S.R.O., Ripley, T.L., Somerville, E.M. et al. Reduced activity at the 5-HT2C receptor enhances reversal learning by decreasing the influence of previously non-rewarded associations. Psychopharmacology 224, 241–254 (2012). https://doi.org/10.1007/s00213-012-2746-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2746-5