Abstract
Rationale
The stop-signal reaction time (SSRT) task measures inhibition of a response that has already been initiated, i.e. the ability to stop. ‘Impulsive’ human subjects, e.g. with attention deficit and hyperactivity disorder (ADHD), have longer SSRTs. Both SSRT and go-trial reaction time (GoRT) may be sensitive to drugs such as d-amphetamine, methylphenidate and modafinil, both in normal subjects and those with ADHD.
Objectives
To investigate the effects of modafinil (3, 10, 30 and 100 mg/kg) and methylphenidate (0.3, 1.0 and 3.0 mg/kg) on SSRT task performance in the rat. To investigate the possible contribution of dopamine receptors in the action of these drugs using the mixed D1/D2 dopamine receptor antagonist cis-flupenthixol.
Results
Modafinil significantly decreased SSRT with little effect on GoRT but only in rats with slow baseline SSRTs. Fast SSRTs were not changed by modafinil. Methylphenidate decreased GoRTs of all rats. However, methylphenidate had baseline-dependent effects on SSRT, decreasing SSRT in slow responders but increasing SSRT in fast responders. Cis-flupenthixol (0.01, 0.04 and 0.125 mg/kg) had no effects on SSRT but increased GoRT at higher doses. At the lowest dose (0.01 mg/kg), cis-flupenthixol failed to disrupt the SSRT-decreasing effects of either modafinil or methylphenidate, whereas at 0.04 mg/kg, the cis-flupenthixol-dependent increase in GoRT was antagonised by methylphenidate but not by modafinil.
Conclusions
This evidence supports a hypothesis that stop and go processes are under control of distinct neurochemical mechanisms.
Similar content being viewed by others
Introduction
The ability to inhibit an ongoing response (stopping) is an important component of behavioural inhibition. The time required to stop a response in this way, the stop-signal reaction time (SSRT), is used extensively as a clinical index of inhibitory control, primarily in the study of attention deficit/hyperactivity disorder (ADHD), where impulsive subjects have slower SSRTs. Disruption of executive functions leading to impaired stopping may be the core deficit in ADHD (Barkley 1997; Turner 2006), although recently, a more integrative model of ADHD has been proposed, which includes stopping as one of several key executive function deficits within ADHD (Castellanos et al. 2006).
Methylphenidate (Ritalin™) is the most commonly prescribed drug in the treatment of ADHD. The monoaminergic neurochemical systems upon which methylphenidate acts include the dopaminergic and noradrenergic systems, both of which have been implicated in modulation of prefrontal cortical function (e.g. Arnsten and Dudley 2005). Positron emission tomography (PET) studies suggest that methylphenidate modulates brain regions associated with motor function to achieve a reduction in ADHD symptoms (Schweitzer et al. 2003). Further PET studies with the dopamine (DA) D2 receptor radioligand [12C] raclopride have shown that orally administered therapeutic doses of methylphenidate elevate DA levels sufficiently to displace striatal D2 receptor occupancy of the ligand (Volkow et al. 2001). Pharmacological evidence suggests that psychostimulants such as methylphenidate and amphetamine act upon the DA system by blocking its reuptake and promoting the release of DA from terminals (Axelrod et al. 1970; Hendley et al. 1972; Ross 1978). The subsequent increase in DA, mainly in the striatum, may underlie the drug’s therapeutic effects. These include an improvement in focussed attention, reduction in impulsive behaviour and a general increase in efficiency of the attentional network system (Rapport et al. 1985b; van der Meere et al. 1995; Zametkin and Borcherding 1989). Empirically, methylphenidate has been shown to reduce SSRT on the stop task in ADHD adults (Aron et al. 2003) and ADHD children (Tannock et al. 1989). However, the effects of methylphenidate in the treatment of ADHD are far from consistent, and there is evidence that in up to 30% of ADHD cases, this drug fails to improve, or even worsens, symptoms such as deficits of impulse control (Cantwell 1996; Krause et al. 2005).
Recently, a non-stimulant drug, modafinil (diphenyl-methyl-sulfinyl-2-acetamide), has gained significant interest as a potential treatment of ADHD. Modafinil is currently used to treat narcolepsy and idiopathic hypersomnia, showing significant therapeutic benefit by stimulating wakefulness and vigilance (Bastuji and Jouvet 1988; Billiard et al. 1994). Modafinil improved symptoms in ADHD children (Rugino and Copley 2001; Rugino and Samsock 2003) and ADHD adults (Taylor and Russo 2000; Turner et al. 2004) and decreased SSRT without incurring effects on the speed of response on go trials in the SSRT task (Turner et al. 2004). In healthy adults, modafinil produced cognitive enhancing effects and decreased SSRT (Turner et al. 2003). Modafinil’s success in the treatment of sleep disorders led to suggestions that the therapeutic benefits conferred to ADHD sufferers may be due to a general improvement in vigilance and attention. However, this has yet to be supported by controlled studies in experimental animals; for example, Waters et al. (2005) found no improvement in rat performance with modafinil on the five-choice serial reaction time test, a well-documented test of attentional control (Robbins 2002).
Turner (2006) concluded that modafinil did not appear to act through a direct dopaminergic mechanism like conventional stimulants (e.g. Lin et al. 1992; Taylor and Russo 2000), although it may activate the locus coeruleus by potentiating tonic DA excitation (Szabadi 2006). Furthermore, the orexin/hypocretin system may be activated via this mechanism (Bubser et al. 2005). Others suggest that the induction of catecholaminergic tone serves to promote histamine release (Ishizuka et al. 2003), or, particularly in the cortical noradrenergic neurones, serotonin-mediated inhibition of γ-amino-n-butyric acid release in the cortex (Tanganelli et al. 1995). Modafinil does not act as a direct agonist of the noradrenergic system (Lin et al. 1992), but nevertheless, its wake-promoting action requires an intact alpha-1 adrenergic system. The effects of modafinil may result from an indirect enhancement of alpha-1 and beta-receptor activity. It is clear that a detailed neuropharmacological profile of action of modafinil is still to be elucidated.
This study directly compared, for the first time, the effects of modafinil and methylphenidate on performance of the rat SSRT task. Rats were classified as either fast or slow responders, where slow responders, with slow SSRTs, reflected deficits found in ADHD and thus could be classified as potentially a more ‘impulsive’ group. d-Amphetamine decreased SSRT in such a slow-responding group (Feola et al. 2000), and we predicted that this slow responding group might also be more responsive to other drugs that improve SSRT-task performance, such as modafinil and methylphenidate. We assessed the involvement of DA in the behavioural effects of both modafinil and methylphenidate by co-administering these drugs with the mixed D1/D2 DA receptor antagonist, cis-flupenthixol.
Materials and methods
Subjects
The subjects were 30 male Lister-hooded rats (Charles River, UK) weighing between 340 and 470 g during experimental testing. Rat weights were approximately 90% of the weights of free-feeding individuals, based on rat growth curves (Harlan, UK). The rats were housed in groups of four animals, in environmentally-enriched cages, under a reversed 12:12 h light–dark cycle (lights off at 07:30), and were tested during the dark phase of this cycle. During behavioural testing, weight gain was restricted (to approximately 1–2 g/week during the main experimental phase) by feeding with a total of 15–20 g of food per day (reinforcer pellets during the task plus laboratory chow given during 1–2 h after the end of the daily test session. Water was freely available except during testing. All experiments were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986.
Apparatus
All sessions were performed in six operant conditioning chambers (Med Associates, Vermont, USA). Each had two retractable levers positioned 70 mm above the chamber floor and 80 mm to either side of a central food well (centre to centre measurement). A houselight in the roof of the chamber remained on throughout the session. A pellet dispenser delivered 45-mg Noyes Formula P pellets (Sandown Scientific, Middlesex, UK) into the food well, and nose entry into the food well was monitored with an infrared detector. A central light, positioned above the food well, signalled reinforcement delivery. Lights above the left and right levers signalled presentation of their respective levers. A 4,500-Hz Sonalert tone generator (Med Associates) was mounted high on the wall opposite to the levers and food well. The chambers were controlled and on-line data were collected by the Whisker control system (Cardinal and Aitken 2001) using the Cambridge Stop Task program written by D. M. Eagle and J. M. C. England.
SSRT task: theoretical issues
The SSRT task is based on the race model (see Logan 1994 for review), which proposes that ‘stop’ and ‘go’ processes are independent of one another, that a ‘race’ occurs between the two processes for completion and that whichever process finishes first wins the race. If the go process wins, a response occurs, and if the stop process wins, a response is inhibited.
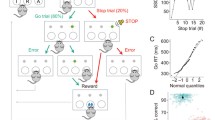
The finishing times of these processes are assumed to vary randomly, so the outcome of the race is a matter of probability. The race model assumes the stop process to be faster than the go process. The position of the stop signal during the go process biases the race in favour of one process or the other. If the signal occurs early in the trial, the response will usually be inhibited (Fig. 1a). Conversely, if the stop signal occurs late enough, the response will rarely be inhibited (Fig. 1b). An inhibition function can be generated between these two extremes by plotting the probability of inhibition against stop-signal delay (SSD; Fig. 1c). An estimate of SSRT can be calculated from the inhibition function and distribution of go-trial reaction times (GoRTs).
(A–C) Representation of the assumptions and predictions of the race model. The probability of inhibition (white shading under the GoRT distribution curve) and the probability of response (black shading under the GoRT distribution curve) for a stop signal either (A) far away from or (B) close to completion of the go response. SSRT = stop-signal reaction time, GoRT = go-trial reaction time, SSD = stop signal delay. (D–H) The stop task for rats. A nose-poke in the central food well begins each trial (D). A press on the left lever begins the ‘go’ response phase of the trial (E). A right lever press (F) is rewarded (G). On ‘stop’ trials, during the response phase of the trial (E), a tone is played. The rat must suppress response on the right lever to attain reward (H). Incorrect responses result in a timeout period
Higher and steeper inhibition functions are indicative of better inhibition processes. If the inhibition process fails to be triggered, or if the stop process is slower than normal, it would lose the race more often, and this would produce a lower and flatter inhibition function. In general, lower and flatter inhibition functions indicate deficits in inhibitory control.
In order for the race model to be applicable to the data, subjects must attempt to perform go trials as quickly as possible while attempting to stop on all trials in which they detect a stop signal. For this reason, the model will fail if a number of precise criteria are not met. Firstly, functions in which subjects nearly always inhibit, or nearly always respond, cannot be interpreted by the race model. Secondly, subjects must not slow the go response in anticipation of the presentation of a stop signal. Subjects that systematically slow GoRT as the stop signal is presented closer to the go response outcome should not be included in the analysis. In the rat SSRT task, trial length is restricted by the limited hold (LH), so lengthening GoRTs may be presented as failed go trials that reflect an increase in successful stop trials. Thirdly, subjects must attempt to stop on all stop trials. Failure to trigger the inhibition process (regardless of the position of the stop signal) would produce a shallower inhibition function. In this case, the highest percentage inhibition achievable would be lower, even with no delay between onset of the go signal and onset of the stop signal. If subjects triggered a stop response on a constant proportion of trials, regardless of the presence or absence of a stop signal, this would be reflected as changes in accuracy of both stop trials and go trials, as the same proportion of go trials would be inhibited as stop trials. Such performance deficits can be monitored in control sessions/trials in which there is no delay to the onset of the stop signal (in which stop- and go-trial accuracy should be at a maximum if the rats are performing the task correctly).
In addition, according to the race model, the shape of the inhibition function also depends on the mean and variability of the ‘go’ process. Increases in both the mean go reaction time (GoRT; may be described as mRT in other studies) and the variability of go reaction times will produce lower and flatter inhibition functions than normal. If the stop-signal presentation is set relative to the mean GoRT rather than to the beginning of the go trial, this may bring disparate inhibition functions into alignment and thus clarify the nature of functional differences between groups. We used this method in our experiment because there was a wide range of mean GoRTs within the group of rats. Resulting differences in the form of the inhibition function are, therefore, likely to be the result of differences in SSRT. Further discussion of this can be found in Logan (1994).
SSRT task: experimental protocol
All rats were trained to perform the SSRT task following a training program that has been previously described in detail (Eagle and Robbins 2003a). Figure 1 shows the stop-task procedure. For training and between-drug baseline sessions, rats received one 20-min session per day, with 200 trials per session. During drug testing, rats received one session per day that was divided into 3 × 10-min test periods, each period with a maximum of 80 trials. In all sessions, trials were initiated with a nose-poke to the central food well, after which, the left lever and left light were presented. A press on the left lever resulted in the right lever and right light being presented, and the left lever and left light were withdrawn/extinguished. If a rat failed to press the left lever within 30 s, the left lever was withdrawn, rats received a 5-s timeout, and the trial was recorded as an omission trial. Rats were trained to perform a rapid reaction time (RT) response from left lever to right lever—the go response. Response speed was maintained by limiting the time for which the right lever was presented—the LH (range 1.0–2.4 s, mean 1.62 ± 0.06 s, maintained at a constant value for each rat throughout the study. Study groups were matched for LH). During go trials, rats were rewarded with a pellet delivered to the central food well for pressing the right lever but received a timeout of 5 s in darkness if they failed to press the right lever within the LH period.
On 20% of the trials, the stop trials, a tone (40 ms, 4,500 Hz) was presented at a predetermined time between the left- and right-lever presses. Stop trials were presented randomly within the session to discourage the rats from anticipating presentation of the stop trials. On stop trials, the rats were required to initiate the same response as on go trials, but after hearing the stop signal, the rat was required to stop completion of the go response, i.e. to refrain from pressing the right lever. The rat was required to withhold from responding for the LH period, after which it was rewarded with a pellet. An incorrect response, which was a press on the right lever, resulted in a timeout of 5 s of darkness. On a few trials designated as stop trials, the rat responded on the right lever before the onset of the tone (more common for late tone presentations), and these trials were reclassified as go trials to maintain the overall proportion of valid stop trials in each session at 20%.
Training and LH
The LH period was set for each rat, during training, at a value that maintained maximum performance accuracy on both stop and go trials (range 1.0–2.4 s). Although there were differences between rats in the LH that would maintain stable and optimal performance, the LH for each rat was maintained at a constant value throughout the study and groups were always matched for LH.
Exclusion criteria and selection procedure
To apply Logan’s race model to behavioural data, rats must perform go trials as quickly as possible while attempting to stop on all stop trials after the stop signal is detected. Failure to perform the task in this way may be reflected in the form of the inhibition function and GoRT across different SSDs. Therefore, rats were excluded from further analysis if they showed inverted inhibition functions (accuracy of stopping improved as the stop signal was played closer to the go signal), if go accuracy was inversely correlated with stop accuracy or if GoRT systematically increased with SSD (more usually presented as a change in go-trial accuracy in the rat SSRT task). Such behavioural patterns reflect strategic changes in performance that cannot be accommodated by the race model. All rats were tested across a full range of SSDs, their inhibition functions were plotted and SSRT calculated.
To investigate inhibition functions after initial training, rats first received 20-min baseline sessions, during which, the stop signal was presented as the left lever was pressed (i.e. with no delay between the onset of the go response and the presentation of the stop signal). Mean GoRT and SSDs for each rat were calculated from three no-delay sessions. Over the following five sessions (20 min, 200 trials), individual rat inhibition functions were generated with SSDs presented in a randomised order from the following set: SSD = GoRT−600 ms; GoRT−500 ms; GoRT−400 ms; GoRT−300 ms; GoRT−200 ms.
From the original group, N = 30, 9 rats were removed because they failed to perform the task according to these criteria set by the race model of SSRT-task performance (Logan 1994; Logan and Cowan 1984). Seven of these rats had inverted inhibition functions and two rats showed go-trial accuracy that inversely correlated with stop-trial accuracy.
Drug-testing protocol
On days where SSRT was calculated (SSRT sessions for drug-testing days and baselines), rats performed one session per day that was divided into 3 × 10-min test periods (StopA, StopB and StopC), each period with a maximum of 80 trials, and with 5 min between test periods, during which, the rats remained in the test chambers. In StopA, on stop trials, there was no delay between the start of the go trial (left lever press) and the onset of the stop signal. Data from StopA were used to calculate mean GoRT for each rat, and this mean GoRT was used to set the SSDs in periods StopB and StopC. StopB presented stop signals at GoRT−500 ms, and Stop C presented stop signals at GoRT−300 ms. Data from GoRT−500 ms and GoRT−300 ms represent points along the steepest section of the rat inhibition function, which provide the most accurate data for calculation of the SSRT. SSRT for each rat was estimated as the mean of estimates calculated from StopB and StopC.
Twenty percent of trials in each test period were randomly determined to be stop trials (16 trials per 80-trial period). During drug testing, drugs were given on Tuesdays and Fridays, with baseline sessions on Mondays and Thursdays. These methods are adapted from the original methods of Eagle and Robbins (2003a). This study used a modified version of drug-testing protocol compared with previous studies (Eagle and Robbins 2003a,b). A comparison of drug-free performance using both the new- and old-method inhibition function data found no significant differences between the old and new methods on SSRT [F(1, 19) = 1.17, n.s.], GoRT [F(1, 19) = 1.95, n.s.], go-trial accuracy [F(1, 19) = 0.83, n.s.] or stop-trial accuracy [F(1, 19) = 0.15, n.s.], so data from old and new studies will be directly comparable. Unless otherwise stated, the level of omissions was less than 1%.
Definition of fast and slow responders
This methodology is based on a study by Feola et al. (2000), in which responsiveness to d-amphetamine treatment was significantly greater in a group with slow SSRTs than a group with fast SSRTs. Subjects with slow SSRTs may more closely resemble subjects with, for example, ADHD, who also may be characterised by their slow SSRTs. If so, we might expect rats with fast or slow SSRTs to have different responses to drugs used in the treatment of ADHD.
The rats were separated into two groups, fast or slow responders, based on their SSRT speed (fast and slow SSRT) on three baseline sessions before drug testing. The median rat was placed in the group that it most closely resembled, giving fast-SSRT group n = 11 and slow-SSRT group n = 10. Selection was based on the mean SSRT across all three baseline sessions, and for all rats, performance in at least two out of the three baseline sessions fell in the correct half of the SSRT distribution. The rank order of SSRTs was significantly different for the fast and the slow groups [SSRT Speed F(1, 19) = 40.74, p < 0.001, mean rank Fast 8.06 ± 0.67, Slow 14.23 ± 0.70], and there was no variation in mean rank across the three baseline sessions (Session × SSRT Speed, n.s.), showing that grouping was stable across the three baseline sessions. Grouping was also stable across the course of the experiment. There was a significant correlation between mean SSRT during the initial baseline sessions and mean SSRT during a baseline measurement at the end of the experimental procedure (Pearson correlation = 0.445, n = 21, p < 0.05).
The behavioural characteristics of the fast- and slow-SSRT groups are summarised in Table 1. There were no differences between the groups in stop-trial accuracy, go-trial accuracy or LH. Although there was a between-group difference in GoRT, there was no significant correlation between SSRT and GoRT on any of the three baseline days of testing (Pearson correlation n.s.).
Drugs
All drugs were made up fresh on the morning of each test day. Modafinil was suspended in methylcellulose (1% w/v methylcellulose in 0.9% saline). Both methylphenidate and cis-flupenthixol were dissolved in sterile 0.9% saline. Doses of methylphenidate and cis-flupenthixol were calculated as the salt in keeping with previous reported use. The pH of cis-flupenthixol solution was adjusted with 6.6 μl/ml 0.1 M NaOH and 3.3 μl/ml 0.1 M HCl to give a pH of 6.4. All drugs were administered intraperitoneally in an injection volume of 1 ml/kg, 30 min before the test session. Where cis-flupenthixol was co-administered with either modafinil or methylphenidate, each drug was administered separately, with cis-flupenthixol administered first and immediately followed by the other drug, injected into a different site i.p.
Experiment 1
In experiment 1, we investigated the effects of modafinil and methylphenidate on stop-task performance. In a previous study (Eagle and Robbins 2003a), we showed that a low dose of d-amphetamine (0.3 mg/kg) significantly improved slow SSRTs, while a higher dose (1.0 mg/kg) disrupted task performance. For this reason, we selected a range of low doses (on a log scale) of both methylphenidate and modafinil for investigation on the SSRT task. After assignment into the fast- and slow-SSRT groups based on baseline performance, each group was further divided into two groups matched for SSRT. One of each of the fast- and slow-SSRT groups received modafinil (0, 3, 10 and 30 mg/kg) and then methylphenidate (0.0, 0.3, 1.0 and 3.0 mg/kg), and the others received methylphenidate then modafinil, according to a Latin-square crossover drug design, with a final dose of modafinil (100 mg/kg) presented as the final drug dose for all rats. Rats received baseline sessions on Mondays and Thursdays and drug on Tuesdays and Fridays. The experiment was completed within 5 weeks. After this experiment, performance was maintained for a period of 3 months before beginning experiment 2.
Experiment 2
In experiment 2, we investigated whether the effects of either modafinil or methylphenidate could be antagonised by the D1/D2 receptor antagonist cis-flupenthixol. Again, we selected a range of low doses of cis-flupenthixol. Experiment 2 was divided into three sections: (a) administration of cis-flupenthixol alone, (b) cis-flupenthixol + modafinil and (c) cis-flupenthixol + methylphenidate. Experimental protocol and drug administration was the same as for experiment 1. Rats received baseline sessions on Mondays and Thursdays and drug on Tuesdays and Fridays. Each of the three sections of the experiment was completed within 2 weeks, with 1–2 weeks between each section.
-
(a)
All rats received cis-flupenthixol at the following doses in order: 0.00, 0.125, 0.04 and 0.01 mg/kg.
For the following experiments, we selected the doses of modafinil and methylphenidate that produced the largest effects on SSRT and investigated the possible antagonistic effects of the two doses of cis-flupenthixol that had the least significant effects alone on the SSRT task.
-
(b)
All rats received drug doses in the following order: modafinil vehicle (1% methylcellulose dissolved in 0.9% saline), modafinil (10 mg/kg), cis-flupenthixol (0.01 mg/kg) + modafinil (10 mg/kg), cis-flupenthixol (0.04 mg/kg) + modafinil (10 mg/kg).
-
(c)
All rats received drug doses in the following order: methylphenidate vehicle (0.9% saline), methylphenidate (1.0 mg/kg), cis-flupenthixol (0.01 mg/kg) + methylphenidate (1.0 mg/kg), cis-flupenthixol (0.04 mg/kg) + methylphenidate (1.0 mg/kg).
After experiment 2 had been completed, performance was maintained for 1 month, after which, a set of three baseline SSRT measurements was taken to compare long-term performance with the initial three baseline sessions.
Data analysis and calculations of SSRT
Data are presented for each set of drug doses plus vehicle. Fast and slow groupings were based on the first SSRT baseline data, which were independent of subsequent analysis and therefore controlled for potential regression to the mean of dose effects. Rats were allocated into fast and slow SSRT groups by a median split of the group, following the protocol defined in Feola et al. (2000). Data collected by the chamber control programmes were compiled in a relational database (Microsoft Access 2000), analysed using SPSS 11.0.1 (SPSS, Chicago, IL). Graphs were plotted using SigmaPlot 8.0 (SPSS) to show group means with error bars of ±1 SEM.
Behavioural data were subjected to repeated-measures analyses of variance (ANOVAs) using a general linear model. All tests of significance were performed at α = 0.05, and models were full factorial unless otherwise stated. SSRT Speed was a between-subject factor and Dose (for single drug, between-dose comparisons) and Drug (for combined drugs) were within-subject variables. Homogeneity of variance was verified using Levene’s test. For repeated-measured analyses, Mauchly’s test of sphericity was applied, and the degrees of freedom corrected to more conservative values using the Huynh–Feldt epsilon ε for any terms involving factors in which the sphericity assumption was violated. Corrected degrees of freedom are corrected to the nearest integer. After repeated-measures analyses, simple one-way ANOVA was used for further analysis, with α adjusted using Sidak’s method α′ = 1−(1 − α)1/c, where c is the number of within-experiment analyses (Howell 1997).
SSRTs were estimated using the protocol described in Logan (1994), from the measures of stop-trial accuracy and the distribution of GoRTs for each rat for each session. RTs on go trials (on which no stop signal occurred) were rank ordered. We selected the nth RT from the ranked list of go-trial RTs, where n was obtained by multiplying the number of RTs in the distribution by the probability of responding on stop trials at that delay. The probability of responding on stop trials was corrected for the presence of omission errors (trials in which the rat failed to respond for a reason unrelated to an increase in SSRT, such as attention or distraction) following a procedure described fully in Eagle and Robbins (2003a). This gave an estimate of the time at which the stopping process finished, relative to the onset of the go signal. To estimate SSRT (the time at which stopping finished relative to the stop signal), the SSD was subtracted from this value.
Results
High-dose drug effects
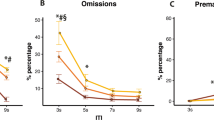
There were significant effects of both modafinil and methylphenidate on the accuracy of performance of go trials [modafinil Dose F(2, 45) = 4.65, p ≤ 0.01; methylphenidate Dose F(2, 33) = 5.53, p ≤ 0.011]. The highest doses of both modafinil (100 mg/kg) and methylphenidate (3.0 mg/kg) significantly disrupted task performance in terms of go-trial accuracy [high dose vs 0.0 mg/kg, Fig. 2a, 100 mg/kg modafinil Dose F(1, 19) = 6.624, p < 0.019, Fig. 2b, 3.0 mg/kg methylphenidate Dose F(1, 19) = 7.092, p < 0.015]. As the estimation of SSRT is based on assumptions that strategy of task performance is not different between groups, these data points are excluded from the further analysis of drug effects on SSRT. There was no significant disruption of go-trial accuracy across all other doses of modafinil (0, 3, 10, 30 mg/kg Dose, n.s.), methylphenidate (0, 0.3, 1.0 mg/kg Dose, n.s.) or at any dose of cis-flupenthixol (Dose, n.s.).
Effects of high dose of drug on baseline task performance. Go-trial accuracy after a modafinil or b methylphenidate. c Trial omissions after cis-flupenthixol. Asterisks significant effect of dose compared to vehicle for either fast- or slow-SSRT groups. Dollar signs significant overall effect of dose compared to vehicle (both fast- and slow-SSRT groups combined). α adjusted from 0.05 using Sidak’s method for multiple comparisons
Although cis-flupenthixol did not affect go-trial accuracy, it did affect the number of trials omitted [trials in which rats failed to press the left lever to start the go phase; Dose F(1, 24) = 12.59, p < 0.001]. The highest dose (0.125 mg/kg) significantly increased the number of omitted trials [Fig. 2c; 0.125 vs 0.0 mg/kg Dose F(1, 19) = 14.98, p < 0.001]. There was no significant disruption of trial initiation across all other doses of cis-flupenthixol (0, 0.01, 0.04 mg/kg Dose, n.s.).
SSRT
Modafinil significantly decreased the SSRT of rats that had slow SSRTs at the beginning of the experiment, but, in contrast, modafinil did not affect the SSRT of rats that had initially fast SSRTs [Fig. 3a; Dose × SSRT Speed F(3, 57) = 2.94, p < 0.05]. The differential effect of modafinil on rats with fast and slow SSRTs was significant for the 10 mg/kg dose [0.0 vs 10.0 mg/kg Dose × SSRT Speed F(1, 19) = 5.94, p ≤ 0.025] and approached significance for the 30 mg/kg dose [0.0 vs 30.0 mg/kg Dose × SSRT Speed F(1, 19) = 4.59, p ≤ 0.045].
Effects of drug dose on SSRT. a Effects of modafinil on SSRT. b Effects of methylphenidate on SSRT. c Effects of methylphenidate on rats with fastest and slowest SSRTs. Vertical bars represent ±SEM. Asterisks significant effect of dose compared to vehicle for either fast- or slow-SSRT groups. Pound/hash sign significant dose × SSRT speed effect compared to vehicle. α adjusted from 0.05 using Sidak’s method for multiple comparisons
Methylphenidate appeared to have no significant effect on SSRT when rats were sub-divided into groups according to their SSRT speed (Fig. 3b; Dose × SSRT Speed, n.s.). However, for this analysis alone, the difference between the performance of the fast- and the slow-SSRT group during vehicle presentation, although still present, was not significant. For this reason, we also analysed the data of the fastest and slowest extremes of each group. When the slowest and fastest extremes of SSRT were investigated (slowest five and fastest five SSRTs, based on baseline performance), there was a significant effect of drug dose on SSRT, with a decrease in SSRT in the slow-SSRT responders, and a significant increase in SSRT in the fast-SSRT responders [Fig. 3c; Dose × SSRT Speed F(2, 16) = 4.57, p < 0.05; 0.0 vs 0.3 mg/kg Dose × SSRT Speed F(1, 8) = 8.27, p ≤ 0.02; 0.0 vs 1.0 mg/kg Dose × SSRT Speed F(1, 8) = 4.52, p ≤ 0.06. For fast-SSRT rats: Dose F(2, 8) = 8.29, p ≤ 0.011, 0.0 vs 0.3 mg/kg Dose F(1, 4) = 13,89, p ≤ 0.02 0.0 vs 1.0 mg/kg Dose F(1, 4) = 13.29, p ≤ 0.022; for slow-SSRT rats: Dose, n.s.].
GoRT
Modafinil had no effect on the GoRT (Fig. 4a; Dose, n.s.; Dose × SSRT Speed, n.s.).
Effects of a modafinil and b methylphenidate on GoRT. Vertical bars represent ±SEM. Asterisks significant effect of dose compared to vehicle for either fast- or slow-SSRT groups. Dollar signs significant overall effect of dose compared to vehicle (both fast- and slow-SSRT groups combined). α adjusted from 0.05 using Sidak’s method for multiple comparisons
Methylphenidate decreased GoRT at all doses [Fig. 4b; Dose F(2, 37) = 5.04, p < 0.025; 0.0 vs 0.3 mg/kg F(1, 19) = 6.96, p ≤ 0.016; 0.0 vs 1.0 mg/kg F(1, 19) = 9.15, p < 0.01]. There was no significant difference between the 0.3 and 1.0 mg/kg doses of methylphenidate, although the higher dose tended to decrease the GoRT more than the lower dose (Dose 0.3 vs 1.0 mg/kg, n.s.). The effect of methylphenidate to decrease GoRT was consistent across all rats (Dose × SSRT Speed, n.s.) but only reached statistical significance in the separate dose–vehicle comparisons for the fast-SSRT group [Fast SSRT 0.0 vs 0.3 mg/kg Dose F(1, 10) = 21.47, p < 0.001; 0.0 vs 1.0 mg/kg Dose F(1, 10) = 10.87, p < 0.01; Slow SSRT 0.0 vs 0.3 mg/kg Dose F(1, 9) = 0.82, n.s.; 0.0 vs 1.0 mg/kg Dose F(1, 9) = 1.92, n.s.].
Cis-flupenthixol
Cis-flupenthixol did not affect SSRT at any dose (Fig. 5a,d; Dose, n.s.), and there was no difference between rats with fast or slow SSRTs in this respect (Dose × SSRT Speed, n.s.). Cis-flupenthixol significantly increased GoRT at 0.04 mg/kg but had no significant effect on GoRT at the lower dose of 0.01 mg/kg [Fig. 6a; Dose F(2, 33) = 3.25, p ≤ 0.05; 0.0 vs 0.01 mg/kg Dose, n.s.; 0.0 vs 0.04 mg/kg Dose F(1, 19) = 7.67, p ≤ 0.012].
Effects of the DA receptor antagonist, cis-flupenthixol, on SSRT. The effects of cis-flupenthixol on SSRT in either a slow-SSRT or d fast-SSRT rats. The effects of cis-flupenthixol and modafinil in either b slow-SSRT or e fast-SSRT rats. The effects of cis-flupenthixol and methylphenidate in either c slow-SSRT or f fast-SSRT rats. Vertical bars represent ±SEM. Asterisks significant effect of dose compared to vehicle for either fast-SSRT or slow-SSRT groups. Pound/hash signs significant dose × SSRT speed effect compared to vehicle (α adjusted from 0.05 using Sidak’s method for multiple comparisons
Effects of the DA receptor antagonist, cis-flupenthixol, on GoRT. a Cis-flupenthixol increased GoRT for all rats. b Modafinil did not interfere with the GoRT-increasing effect of cis-flupenthixol. c Methylphenidate prevented the GoRT-increasing effect of cis-flupenthixol. Vertical bars represent ±SEM. Dollar signs significant overall effect of dose compared to vehicle (both fast- and slow-SSRT groups combined). § significant difference for specified comparison. adjusted from 0.05 using Sidak’s method for multiple comparisons
Combined effect of cis-flupenthixol + modafinil on SSRT
Cis-flupenthixol, at a dose of 0.01 mg/kg that did not induce any observable effects on either SSRT or GoRT, failed to antagonise the SSRT-decreasing effects of modafinil (Fig. 5b). There was, once again, a strong SSRT-decreasing effect of 10 mg/kg modafinil for rats with slow SSRTs [vehicle vs modafinil 10 mg/kg, Dose F(1, 19) = 7.33, p < 0.025, Dose × SSRT Speed F(1, 19) = 6.42, p < 0.025; Fast SSRT Dose, n.s., Slow SSRT Dose F(1, 9) = 9.01, p ≤ 0.015]. This SSRT-decreasing effect of modafinil in the slow-SSRT rats was maintained when rats received cis-flupenthixol + modafinil at both the 0.01 mg/kg and also at the behaviourally-disruptive 0.04 mg/kg dose, that is, there were no significant differences in SSRT between the modafinil treatment and the cis-flupenthixol + modafinil treatment (modafinil 10 mg/kg vs cis-flupenthixol 0.01 mg/kg + modafinil 10 mg/kg, Drug, n.s.; modafinil 10 mg/kg vs cis-flupenthixol 0.04 mg/kg + modafinil 10 mg/kg, Drug, n.s.). Treatment with cis-flupenthixol + modafinil was thus significantly different from treatment with vehicle alone [vehicle vs cis-flupenthixol 0.01 mg/kg + modafinil 10 mg/kg Drug F(1, 19) = 6.95, p < 0.025; cis-flupenthixol 0.04 mg/kg + modafinil Drug F(1, 19) = 9.21, p < 0.01]. For the rats with fast SSRTs, although there appeared to be a slight decrease in SSRT after cis-flupenthixol + modafinil compared with the effects of modafinil alone, this effect was not significant (Fast SSRT Drug, n.s.).
Combined effect of cis-flupenthixol + modafinil on GoRT
Modafinil failed to antagonise the GoRT-increasing effects of the higher dose of cis-flupenthixol (Fig. 6b; 0.04 mg/kg cis-flupenthixol vs 0.04 mg/kg cis-flupenthixol + 10 mg/kg modafinil Drug, n.s.). All rats had significantly increased GoRTs after 0.04 mg/kg cis-flupenthixol + modafinil than when treated with modafinil alone [modafinil 10 mg/kg vs cis-flupenthixol 0.04 mg/kg + modafinil 10 mg/kg Drug F(1, 19) = 11.15, p < 0.01]. Treatment with 0.01 mg/kg cis-flupenthixol and modafinil had no significant effects on GoRT (0.01 mg/kg cis-flupenthixol vs 0.01 mg/kg cis-flupenthixol + 10 mg/kg modafinil Drug, n.s.).
Combined effect of cis-flupenthixol + methylphenidate on SSRT
Cis-flupenthixol at a dose of 0.01 mg/kg failed to affect the action of methylphenidate on SSRT (Fig. 5c,f). Methylphenidate differentially affected SSRTs of rats with slow or fast initial SSRTs [vehicle vs methylphenidate 1.0 mg/kg Dose × SSRT Speed F(1, 18) = 6.91, p ≤ 0.017], although only the SSRT-increasing effect of methylphenidate in fast-SSRT rats, but not the SSRT-decreasing effect of methylphenidate in slow-SSRT rats, was independently significant [slow-SSRT, vehicle vs methylphenidate Dose, n.s.; fast-SSRT, vehicle vs methylphenidate Dose F(1, 10) = 11.43, p < 0.01]. Neither dose of cis-flupenthixol + methylphenidate significantly changed SSRT compared to treatment with methylphenidate alone (Fast-SSRT rats; methylphenidate 1.0 mg/kg vs cis-flupenthixol 0.01 mg/kg + methylphenidate 1.0 mg/kg Drug, n.s.; methylphenidate 1.0 mg/kg vs cis-flupenthixol 0.04 mg/kg + methylphenidate 1.0 mg/kg Drug, n.s; Slow-SSRT rats; methylphenidate 1.0 mg/kg vs cis-flupenthixol 0.01 mg/kg + methylphenidate 1.0 mg/kg Drug, n.s.; methylphenidate 1.0 mg/kg vs cis-flupenthixol 0.04 mg/kg + methylphenidate 1.0 mg/kg Drug, n.s.).
Combined effect of cis-flupenthixol + methylphenidate on GoRT
Methylphenidate significantly antagonised the GoRT-increasing effects of the higher dose of cis-flupenthixol [Fig. 6c; 0.04 mg/kg cis-flupenthixol vs 0.04 mg/kg cis-flupenthixol + 1.0 mg/kg methylphenidate Drug F(1, 19) = 4.95, p < 0.05]. Treatment with 0.04 mg/kg cis-flupenthixol + methylphenidate failed to produce significant increase in GoRT compared to treatment with methylphenidate alone (for all rats Drug, n.s.; Drug × SSRT Speed, n.s.).
Discussion
This study has shown distinct differences in the effects of modafinil and methylphenidate on the SSRT task in rats, drugs that have proven efficacy in the treatment of ADHD and that decrease SSRT in this task. The effects of both drugs on SSRT-task performance are consistent with their effects in human subjects.
As in human subjects, modafinil consistently improved inhibitory response control throughout the study but in a baseline-dependent manner. Modafinil decreased the SSRT of rats with high-baseline (slow) SSRTs, whereas it had little effect on the SSRTs of rats with low-baseline (fast) SSRTs. Further observation of these data showed that in some cases, after 10 and 30 mg/kg modafinil, the SSRTs of slow-SSRT rats became faster than the SSRTs of fast-SSRT rats, which implies that the lack of effect of modafinil on the fast-SSRT group was not a result of a floor effect on performance. Modafinil did not affect the GoRT in any rats, and at the doses of modafinil that decreased SSRT, there were no significant changes in baseline (no delay) go- or stop-trial accuracy. This implies that the effects of modafinil within the range up to 30 mg/kg were confined specifically to the speed of the stop process rather than to more fundamental processes of attention or response selection, which would have been evident as changes in accuracy of the stop or go responses during no-delay trials. The highest dose of modafinil (100 mg/kg), however, did reduce go-trial performance accuracy in no-delay trials, suggesting that this dose of modafinil had effects on attention or response selection. Such deficits cannot be interpreted using the race model and disqualify these data from inclusion in any SSRT analysis using the race model. This shows, however, that our study tested modafinil across the full range of doses that were practical for assessment of the effects of this drug on SSRT. All effects of modafinil were highly consistent between experiments 1 and 2.
Methylphenidate consistently decreased GoRT throughout the study, however, its effects on SSRT were less straightforward. In general, methylphenidate tended to decrease SSRT of slow responders whereas increasing SSRT of fast responders, but the effects of this drug were less consistent across experiments. There was no overall dose effect of methylphenidate in experiment 1, but, in the vehicle-treatment condition for this experiment alone, the normally clear-cut difference between rats with fast and slow SSRTs, while still present, was not significant. Analysis of our groupings of rats by SSRT speed, across both the full course of the experiment and between individual baseline sessions, has shown that this grouping is normally highly robust over time, and we were unable to explain this lack of difference between groups in the vehicle-treatment condition. For this data set, we selected rats with the most extreme slow- and fast-SSRT baseline performance for further analysis. This recovered the difference between slow- and fast-SSRT rats, and those with the slowest SSRTs showed a decrease in SSRT when treated with methylphenidate, whereas the rats with the fastest SSRTs showed an increase in SSRT with methylphenidate. This baseline-dependent pattern of response to methylphenidate was exhibited by the whole group during experiment 2. Like modafinil, the highest dose tested during this study (3.0 mg/kg) disrupted go-trial performance by reducing accuracy of go trials in no-delay sessions, rendering the race model inapplicable. Again, this suggests that our study tested methylphenidate across the full range of doses that were suitable for assessment of this drug on SSRT.
Both modafinil and methylphenidate produced baseline-dependent effects on SSRT that were similar to the effects of d-amphetamine, which also had baseline-dependent effects on SSRT, although it produced no significant effects on the population as a whole (Feola et al. 2000; Fillmore et al. 2005). The use of normal variation within a population to investigate disorders such as ADHD is valuable, as we can model symptoms of these disorders while making no assumptions about their underlying pathology, unlike other potential ‘models’ of ADHD.
We tested whether drug-dependent changes in task performance could be attenuated by treatment with the mixed D1/D2 DA receptor antagonist cis-flupenthixol. Cis-flupenthixol by itself had no significant effects on SSRT at a dose (0.04 mg/kg) that significantly increased GoRT. The highest dose of cis-flupenthixol tested during this study resulted in rats failing to initiate trials (increased trial omissions), which again suggested that we investigated the effects of cis-flupenthixol over a range of doses that were suitable for assessment of the effects of this drug on SSRT.
Cis-flupenthixol failed to block the SSRT-changing properties of either modafinil or methylphenidate. This implies that the actions of neither modafinil nor methylphenidate on SSRT were mediated directly via DA D1 or D2 receptors. This is further supported by the evidence that cis-flupenthixol alone had no effect on SSRT in either direction. However, modafinil and methylphenidate showed different interactions with cis-flupenthixol on GoRT. Modafinil did not affect the GoRT-increasing effects of cis-flupenthixol. In comparison, when rats were treated with methylphenidate and cis-flupenthixol together, the overall result was a decrease in GoRT (i.e. the DA-receptor-antagonist-increase in GoRT was blocked by the action of methylphenidate). This suggests that the methylphenidate-induced decrease and the cis-flupenthixol-induced increase in GoRT are the result of effects on the DA system.
Although it is possible that either or both modafinil or methylphenidate might act via other DA receptors, there is no clear evidence to support a dopaminergic mechanism of SSRT control. Although polymorphisms in the DA receptor D4 (DRD4) gene in ADHD are though to be critical for cognitive function in some respects, a comparison of ADHD children, with or without at least one DRD4 7-repeat allele, found no difference in stopping behaviour, although there was a difference in GoRTs (Langley et al. 2004). Our results are also in agreement with other recent data showing a lack of effect of l-DOPA on SSRT (Overtoom et al. 2003). Altogether, the evidence, at present, is against a direct role for DA in the stopping process.
These results provide support for a hypothesis that the stop and go processes are, at least partly, under the control of different neurotransmitter systems. A recent study of atomoxetine treatment on the SSRT task implicated noradrenaline in the SSRT process, noradrenaline function within the prefrontal cortex being critical for several forms of behavioural control including inhibition (Arnsten and Dudley 2005; Arnsten and Li 2005), but not the GoRT process (Chamberlain et al. 2006). Atomoxetine also decreased SSRT in rats (Robinson et al. 2006). It appears that in general, reaction time processes are susceptible to dopaminergic manipulations (e.g. Arnsten and Li 2005; Gauntlett-Gilbert and Brown 1998; Lalonde and Botez-Marquard 1997; Robbins 2002), whereas stopping processes are not. This adds support for a hypothesis of differential neurochemical control within the SSRT task, whereby noradrenaline is more critical for the stop process, but DA is more critical for the go process.
The effect of modafinil in behavioural control
The effects of modafinil on rat SSRT-task performance are directly comparable with the effects of this drug in both healthy human subjects and those with ADHD (e.g. Aron et al. 2003; Tannock et al. 1989; Turner et al. 2003, 2004), producing a selective improvement in SSRT without significant effects on GoRT. Although only those rats with slow SSRTs showed a decrease in SSRT with modafinil, there is evidence from the human studies that subjects with the slowest SSRTs show greater improvements after modafinil treatment than those with the fastest SSRTs (Turner, personal communication).
Modafinil improves cognitive ability on a number of measures of executive function as well as on behavioural inhibition or stopping. While these cognitive enhancements are often related to the more extensively-documented wake-promoting effects of modafinil (e.g. Dagan and Doljansky 2006; Gill et al. 2006), it is becoming clear that modafinil may also improve cognitive ability in non-sleep-deprived subjects. For example, modafinil significantly improved the performance of healthy adults in tests of visual pattern recognition memory, delayed matching-to-sample, digit span and spatial planning (Turner et al. 2003). In preclinical tests, modafinil may also improve cognitive functions in mice and rats. For example, modafinil improved learning in a delayed non-matching to position task (Ward et al. 2004). However, other studies of both human and rodent subjects have failed to find cognitive-enhancing effects of modafinil (e.g. Randall et al. 2003; Waters et al. 2005).
Although the exact nature of the effects of modafinil on cognitive function requires further investigation, our study suggests that the effect of modafinil on stopping behaviour is robust, and that low, rather than high, doses of modafinil may be more effective in improving certain deficiencies in behavioural function.
The effect of methylphenidate in behavioural control
Although methylphenidate is a common treatment for symptoms of ADHD, both in children and adults, in up to 30% of cases, methylphenidate failed to improve ADHD symptoms and often increased SSRT in these subjects (Cantwell 1996; Krause et al. 2005). Non-responders may be more prevalent among older ADHD subjects, and there are also reports that children with co-morbid anxiety/internalising disorders are less likely to respond well to methylphenidate (Boonstra et al. 2005; Buitelaar et al. 1995; DuPaul et al. 1994; Pliszka 1989; Rapport et al. 1985a; Tannock et al. 1995; Taylor et al. 1987). The subset of non-responders showed differences in event-related potentials when on medication; non-responders had significantly longer N2 and P3b latencies than responders (Sunohara et al. 1997, 1999), and there is evidence of morphological differences between responders and non-responders to methylphenidate (Semrud-Clikeman et al. 1994) as well as in levels of DA transporter (Krause et al. 2005).
The effects of methylphenidate on rat SSRT-task performance were consistent with many of the human studies. As we found with modafinil, changes in SSRT were not only baseline-dependent, but SSRT was increased in initially fast-responding rats. This increase in SSRT for rats with fast SSRTs might reflect the behaviour of ‘non-responders’ to methylphenidate in the human population. SSRT only improved slightly in subjects with slow baseline SSRTs, an effect matched by a recent study of adult ADHD in which only subjects with slow SSRTs were improved by methylphenidate on a stop-change task (Boonstra et al. 2005). Further direct comparison of this effect with human studies is not yet possible because human SSRT studies rarely report individual changes in performance after drug treatments. Nevertheless, as interest continues to focus on between and within subject variability in ADHD, investigation of baseline behavioural characteristics of methylphenidate responders and non-responders could provide important insight into the mechanism of action of this drug in ameliorating symptoms of ADHD.
We found that methylphenidate did not show specificity for the stop process, in agreement with a number of human studies that showed decreases in both SSRT and GoRT after methylphenidate treatment (e.g. Bedard et al. 2003; Lijffijt et al. 2006; Scheres et al. 2003; Tannock et al. 1989: for exceptions, see Aron et al. 2003, where methylphenidate improved SSRT but not GoRT in adult ADHD and Overtoom et al. 2003, where methylphenidate improved GoRT but not SSRT in childhood ADHD). As a treatment for deficits in SSRT task performance, methylphenidate appears to have a range of effects that are not specific to SSRT per se. This means that in subjects, for example in adult ADHD, for whom the only deficit in SSRT-task performance is an increased SSRT, methylphenidate may not be the most effective treatment as its lack of specificity may produce undesirable side effects (e.g. decreases in otherwise-normal GoRTs).
In summary, both modafinil and methylphenidate improved performance on a SSRT task, although the effects of modafinil were specific to the stopping process, whereas methylphenidate had effects that generalised to both stop and go processes. The effectiveness of both drugs on SSRT was baseline-dependent. Combined treatment with the mixed D1/D2 receptor antagonist, cis-flupenthixol, suggested that the effects of both modafinil and methylphenidate on stopping were independent of DA. In contrast, DA manipulations significantly influenced the go process. This evidence supports the independence of the stop and go processes and implies control by distinct neurotransmitter mechanisms, which has implications for the use of both methylphenidate and modafinil in the treatment of this particular form of behavioural disinhibition.
References
Arnsten AF, Dudley AG (2005) Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Funct 1:2
Arnsten AF, Li BM (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384
Aron AR, Dowson JH, Sahakian BJ, Robbins TW (2003) Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 54:1465–1468
Axelrod J, Mueller RA, Henry JP, Stephens PM (1970) Changes in enzymes involved in the biosynthesis and metabolism of noradrenaline and adrenaline after psychosocial stimulation. Nature 225:1059–1060
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94
Bastuji H, Jouvet M (1988) Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuro-psychopharmacol Biol Psychiatry 12:695–700
Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R (2003) Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol 31:315–327
Billiard M, Besset A, Montplaisir J, Laffont F, Goldenberg F, Weill JS, Lubin S (1994) Modafinil: a double-blind multicentric study. Sleep 17:S107–S112
Boonstra AM, Kooij JJ, Oosterlaan J, Sergeant JA, Buitelaar JK (2005) Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? J Clin Exp Neuropsychol 27:278–298
Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY (2005) Dopaminergic regulation of orexin neurons. Eur J Neurosci 21:2993–3001
Buitelaar JK, Van der Gaag RJ, Swaab-Barneveld H, Kuiper M (1995) Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34:1025–1032
Cantwell DP (1996) Attention deficit disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 35:978–987
Cardinal RN, Aitken MRF (2001) Whisker, version 2.2, computer software
Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R (2006) Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci 10:117–123
Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863
Dagan Y, Doljansky JT (2006) Cognitive performance during sustained wakefulness: a low dose of caffeine is equally effective as modafinil in alleviating the nocturnal decline. Chronobiol Int 23:973–983
DuPaul GJ, Barkley RA, McMurray MB (1994) Response of children with ADHD to methylphenidate: interaction with internalizing symptoms. J Am Acad Child Adolesc Psychiatry 33:894–903
Eagle DM, Robbins TW (2003a) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317
Eagle DM, Robbins TW (2003b) Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res 146:131–144
Feola TW, de Wit H, Richards JB (2000) Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci 114:838–848
Fillmore MT, Kelly TH, Martin CA (2005) Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend 77:151–159
Gauntlett-Gilbert J, Brown VJ (1998) Reaction time deficits and Parkinson’s disease. Neurosci Biobehav Rev 22:865–881
Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA (2006) Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med 13:158–165
Hendley ED, Snyder SH, Fauley JJ, LaPidus JB (1972) Stereoselectivity of catecholamine uptake by brain synaptosomes: studies with ephedrine, methylphenidate and phenyl-2-piperidyl carbinol. J Pharmacol Exp Ther 183:103–116
Howell DC (1997) Statistical methods for psychology, 4th edn. Duxbury, Belmont
Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A (2003) Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett 339:143–146
Krause J, la Fougere C, Krause KH, Ackenheil M, Dresel SH (2005) Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci 255:428–431
Lalonde R, Botez-Marquard T (1997) The neurobiological basis of movement initiation. Rev Neurosci 8:35–54
Langley K, Marshall L, van den Bree M, Thomas H, Owen M, O’Donovan M, Thapar A (2004) Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry 161:133–138
Lijffijt M, Kenemans JL, Wal AT, Quik EH, Kemner C, Westenberg H, Verbaten MN, Engeland HV (2006) Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/hyperactivity disorder. Eur Psychiatr 21:544–547
Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M (1992) Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res 591:319–326
Logan GD (1994) On the ability to inhibit thought and action. A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH (eds) Inhibitory processes in attention, memory and language. Academic, San Diego, CA, pp 189–236
Logan GD, Cowan WB (1984) On the ability to inhibit thought and action—a theory of an act of control. Psychol Rev 91:295–327
Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, van der Molen MW, van der Gugten J, Westenberg H, Maes RA, Koelega HS (2003) Effects of methylphenidate, desipramine, and l-dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behav Brain Res 145:7–15
Pliszka SR (1989) Effect of anxiety on cognition, behavior, and stimulant response in ADHD. J Am Acad Child Adolesc Psychiatry 28:882–887
Randall DC, Shneerson JM, Plaha KK, File SE (2003) Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol 18:163–173
Rapport MD, DuPaul GJ, Stoner G, Birmingham BK, Masse G (1985a) Attention deficit disorder with hyperactivity: differential effects of methylphenidate on impulsivity. Pediatrics 76:938–943
Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S (1985b) Methylphenidate in hyperactive children: differential effects of dose on academic, learning, and social behavior. J Abnorm Child Psychol 13:227–243
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Robinson ESJ, Eagle DM, Banerjee G, Jiang X, Robbins TW (2006) Effects of atomoxetine on inhibitory control in the rat stop signal task. J Psychopharmacol 20:A67
Ross SB (1978) Antagonism by methylphenidate of the stereotyped behaviour produced by (+)-amphetamine in reserpinized rats. J Pharm Pharmacol 30:253–254
Rugino TA, Copley TC (2001) Effects of modafinil in children with attention-deficit/hyperactivity disorder: an open-label study. J Am Acad Child Adolesc Psychiatry 40:230–235
Rugino TA, Samsock TC (2003) Modafinil in children with attention-deficit hyperactivity disorder. Pediatr Neurol 29:136–142
Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant JA (2003) The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol 31:105–120
Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, Kilts CD (2003) A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology 28:967–973
Semrud-Clikeman M, Filipek PA, Biederman J, Steingard R, Kennedy D, Renshaw P, Bekken K (1994) Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry 33:875–881
Sunohara GA, Voros JG, Malone MA, Taylor MJ (1997) Effects of methylphenidate in children with attention deficit hyperactivity disorder: a comparison of event-related potentials between medication responders and non-responders. Int J Psychophysiol 27:9–14
Sunohara GA, Malone MA, Rovet J, Humphries T, Roberts W, Taylor MJ (1999) Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacology 21:218–228
Szabadi E (2006) Drugs for sleep disorders: mechanisms and therapeutic prospects. Br J Clin Pharmacol 61:761–766
Tanganelli S, Perez de la Mora M, Ferraro L, Mendez-Franco J, Beani L, Rambert FA, Fuxe K (1995) Modafinil and cortical gamma-aminobutyric acid outflow. Modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol 273:63–71
Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD (1989) Effects of methylphenidate on inhibitory control in hyperactive-children. J Abnorm Child Psychol 17:473–491
Tannock R, Ickowicz A, Schachar R (1995) Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. J Am Acad Child Adolesc Psychiatry 34:886–896
Taylor FB, Russo J (2000) Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol 10:311–320
Taylor E, Schachar R, Thorley G, Wieselberg HM, Everitt B, Rutter M (1987) Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behaviour. Psychol Med 17:121–143
Turner D (2006) A review of the use of modafinil for attention-deficit hyperactivity disorder. Expert Rev Neurother 6:455–468
Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ (2003) Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology 165:260–269
Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ (2004) Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 55:1031–1040
van der Meere J, Shalev R, Borger N, Gross-Tsur V (1995) Sustained attention, activation and MPH in ADHD: a research note. J Child Psychol Psychiatry 36:697–703
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21:RC121
Ward CP, Harsh JR, York KM, Stewart KL, McCoy JG (2004) Modafinil facilitates performance on a delayed nonmatching to position swim task in rats. Pharmacol Biochem Behav 78:735–741
Waters KA, Burnham KE, O’Connor D, Dawson GR, Dias R (2005) Assessment of modafinil on attentional processes in a five-choice serial reaction time test in the rat. J Psychopharmacol 19:149–158
Zametkin AJ, Borcherding BG (1989) The neuropharmacology of attention-deficit hyperactivity disorder. Annu Rev Med 40:447–451
Acknowledgements
This study was supported by a Wellcome Trust Program Grant awarded to TWR, B. J. Everitt, A. C. Roberts and B. J. Sahakian, and completed within the University of Cambridge Behavioural and Clinical Neuroscience Institute, supported by a joint award from Medical Research Council and the Wellcome Trust. All procedures comply with the current laws of the UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eagle, D.M., Tufft, M.R.A., Goodchild, H.L. et al. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology 192, 193–206 (2007). https://doi.org/10.1007/s00213-007-0701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0701-7