Abstract
Rationale
Although imaging studies in human addicts have been valuable for identifying the neural substrates of the effects of abused drugs, few studies have used this approach in animal models where conditions can be carefully controlled.
Objective
To define the substrates that mediate the effects of cocaine in a rodent model of cocaine self-administration using the 2-[14C]deoxyglucose method and to assess changes in these patterns over the course of drug exposure.
Methods
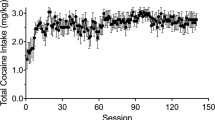
Male Sprague-Dawley rats self-administered cocaine (0.75 mg/kg per injection; FR2; 21 injections/session) and control rats received saline infusions in the same pattern as the cocaine rats for 5 or 30 days. Metabolic mapping was applied immediately after the final session.
Results
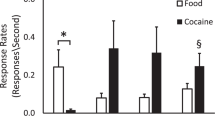
Following 5 days of self-administration, rates of glucose utilization were decreased in the nucleus accumbens, and increased in autonomic brainstem structures and in sensorimotor regions. After 30 days of cocaine exposure, self-administration reduced glucose utilization throughout the dorsal and ventral striatum, central nucleus of the amygdala, medial forebrain bundle, and infralimbic and prelimbic prefrontal cortices. In addition, at this time point glucose utilization was no longer elevated in any autonomic or sensorimotor brain regions.
Conclusions
These data demonstrate that the distribution of functional activity associated with self-administered cocaine undergoes considerable change over the course of drug exposure. While increases in metabolic rates were largely found in autonomic and sensorimotor structures after short-term cocaine access, decreases were prominent in mesocorticolimbic regions after prolonged exposure. These differences in the patterns of brain activity that develop with long-term cocaine self-administration may play a role in the transition to habitual drug seeking behavior.


Similar content being viewed by others
References
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
Alheid G, DeOlmos JS, Beltramino CA (1995) Amygdala and extended amygdala. In: Paxinos G (ed) The rat nervous system. Academic Press, New York, pp 495–578
Ambrosio E, Tella SR, Goldberg SR, Schindler CW, Erzouki H, Elmer GI (1996) Cardiovascular effects of cocaine during operant cocaine self-administration. Eur J Pharmacol 315:43–51
Borowsky B, Kuhn CM (1991) Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther 256:204–210
Crane AM, Porrino LJ (1989) Adaptation of the quantitative 2-[14C]deoxyglucose method for use in freely moving rats. Brain Res 499:87–92
Crespo JA, Manzanares J, Oliva JM, Corchero J, Palomo T, Ambrosio E (2001) Extinction of cocaine self-administration produces a differential time-related regulation of proenkephalin gene expression in rat brain. Neuropsychopharmacology 25:185–194
Dworkin SI, Smith JE (1988) Neurobehavioral pharmacology of cocaine. NIDA Res Monogr 88:185–198
Dworkin SI, Porrino LJ, Smith JE (1992) Importance of behavioral controls in the analysis of ongoing events. In: Brown RM, Frascella J (eds) Neurobiological approaches to brain-behavior interaction. NIDA Monograph, pp 173–218
Dworkin SI, Co C, Smith JE (1995) Rat brain neurotransmitter turnover rates altered during withdrawal from chronic cocaine administration. Brain Res 682:116–126
Erb S, Salmaso N, Rodaros D, Stewart J (2001) A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology 158:360–365
Fischman MW, Schuster CR, Resnekov L, Shick JF, Krasnegor NA, Fennell W, Freedman DX (1976) Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry 33:983–989
Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD (1988) Repeated intranasal cocaine administration: lack of tolerance to pressor effects. Drug Alcohol Depend 22:169–177
Galici R, Pechnick RN, Poland RE, France CP (2000) Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol 387:59–62
Goeders NE (1997) A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology 22:237–259
Goeders NE, Guerin GF (1996) Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res 722:145–152
Graham J, Porrino LJ (1995) Neuroanatomical substrates of cocaine self-administration. In: Hammer R (ed) Neurobiology of cocaine. CRC Press, Boca Raton, pp 3–14
Hammer RP Jr, Cooke ES (1994) Gradual tolerance of metabolic activity is produced in mesolimbic regions by chronic cocaine treatment, while subsequent cocaine challenge activates extrapyramidal regions of rat brain. J Neurosci 14:4289–4298
Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI (1997) Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology 133:7–16
Ito R, Dalley JW, Robbins TW, Everitt BJ (2002) Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22:6247–6253
Kiyatkin EA, Stein EA (1995) Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience 64:599–617
Knapp CM, Printseva B, Cottam N, Kornetsky C (2002) Effects of cue exposure on brain glucose utilization 8 days after repeated cocaine administration. Brain Res 950:119–126
Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13:177–184
Lau CE, Sun L (2002) The pharmacokinetic determinants of the frequency and pattern of intravenous cocaine self-administration in rats by pharmacokinetic modeling. Drug Metab Dispos 30:254–261
Leri F, Flores J, Rodaros D, Stewart J (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–5718
Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ (2001) Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci 21:2799–2807
Loewy A, Mckeller S (1980) The neuroanatomical basis of central cardiovascular control. Fed Proc 39:2945–2503
London ED, Wilkerson G, Goldberg SR, Risner ME (1986) Effects of l-cocaine on local cerebral glucose utilization in the rat. Neurosci Lett 68:73–78
Macey DJ, Smith HR, Nader MA, Porrino LJ (2003) Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci 23:12–16
Mackler SA, Eberwine JH (1991) The molecular biology of addictive drugs. Mol Neurobiol 5:45–58
Markou A, Koob GF (1991) Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26
Mello NK, Mendelson JH (1997) Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav 57:571–599
Mendelson JH, Teoh SK, Lukas SE, Phipps W, Ellingboe J, Palmieri SL, Schiff I (1992) Human studies on the biological basis of reinforcement: a neuroendocrine perspective. In: Jaffe JH (ed) Addictive states. Raven Press, New York, pp 131–155
Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J (2002) Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinology 27:71–82
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, New York
Piazza PV, Le Moal M (1998) The role of stress in drug self-administration. Trends Pharmacol Sci 19:67–74
Pitts DK, Marwah J (1986) Electrophysiological effects of cocaine on central monoaminergic neurons. Eur J Pharmacol 131:95–98
Pontieri FE, Colangelo V, La Riccia M, Pozzilli C, Passarelli F, Orzi F (1994) Psychostimulant drugs increase glucose utilization in the shell of the rat nucleus accumbens. Neuroreport 5:2561–2564
Pontieri FE, Tanda G, Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA 92:12304–12308
Porrino LJ (1993) Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology 112:343–351
Porrino LJ, Lucignani G, Dow-Edwards D, Sokoloff L (1984) Correlation of dose-dependent effects of acute amphetamine administration on behavior and local cerebral metabolism in rats. Brain Res 307:311–320
Porrino LJ, Domer FR, Crane AM, Sokoloff L (1988) Selective alterations in cerebral metabolism within the mesocorticolimbic dopaminergic system produced by acute cocaine administration in rats. Neuropsychopharmacology 1:109–118
Porrino LJ, Lyons D, Letchworth SR, Freedland CS, Nader MA (2002) Structural and functional neuroimaging of the effects of cocaine in human and nonhuman primates. In: Massaro EJ (ed) Handbook of neurotoxicology. Humana Press, Totowa, N.J., pp 413–435
Resnick RB, Kestenbaum RS, Schwartz LK (1977) Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science 195:696–698
Roberts DC, Koob GF, Klonoff P, Fibiger HC (1980) Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12:781–787
Roberts DC, Brebner K, Vincler M, Lynch WJ (2002) Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend 67:291–299
Shaham Y, Erb S, Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Stein EA, Fuller SA (1992) Selective effects of cocaine on regional cerebral blood flow in the rat. J Pharmacol Exp Ther 262:327–334
Tella SR, Schindler CW, Goldberg SR (1992) Cardiovascular effects of cocaine in conscious rats: relative significance of central sympathetic stimulation and peripheral neuronal monoamine uptake and release mechanisms. J Pharmacol Exp Ther 262:602–610
Tella SR, Schindler CW, Goldberg SR (1999) Cardiovascular responses to cocaine self-administration: acute and chronic tolerance. Eur J Pharmacol 383:57–68
Thomas WL Jr, Cooke ES, Hammer RP Jr (1996) Cocaine-induced sensitization of metabolic activity in extrapyramidal circuits involves prior dopamine D1-like receptor stimulation. J Pharmacol Exp Ther 278:347–353
Tornatzky W, Miczek KA (1999) Repeated limited access to IV cocaine self-administration: conditioned autonomic rhythmicity illustrating “predictive homeostasis”. Psychopharmacology 145:144–152
Volkow ND, Fowler JS (2000) Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10:318–325
Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M (2002) The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem 82:1453–1464
Zocchi A, Conti G, Orzi F (2001) Differential effects of cocaine on local cerebral glucose utilization in the mouse and in the rat. Neurosci Lett 306:177–180
Acknowledgements
This research was funded by the National Institutes of Drug Abuse grants DA 07246 (D.M.) and DA 06634 (L.J.P.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macey, D.J., Rice, W.N., Freedland, C.S. et al. Patterns of functional activity associated with cocaine self-administration in the rat change over time. Psychopharmacology 172, 384–392 (2004). https://doi.org/10.1007/s00213-003-1676-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1676-7