Summary
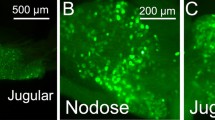
Immunoreactivity to the rate limiting enzyme of catecholamine synthesis, tyrosine hydroxylase, has been described in the inferior sensory (= nodose) ganglion of the vagal nerve in the rat. The aim of the present study was to characterize further this neuronal population. The neurons do not represent displaced autonomic efferent neurons, since they do not receive synaptic input, as indicated by the absence of synaptophysin-immunoreactive terminals. In addition to the immunoreactivity to tyrosine hydroxylase, a tyrosine hydroxylase cRNA probe hybridizes with nodose ganglion neurons as demonstrated by in situ hybridization and Northern blotting. Many but not all of the tyrosine hydroxylase-immunoreactive neurons are also immunoreactive to the dopamine synthesizing enzyme, aromatic-l-amino-acid-decarboxylase, but lack the noradrenaline-synthesizing enzyme, dopamine-β-hydroxylase, thus favoring synthesis of dopamine. Neuropeptide Y, which is often colocalized with catecholamines, is also present in a subset of nodose ganglion neurons, as indicated by immunohistochemistry, in situ hybridization and Northern blotting. However, double-labeling immunofluorescence has revealed that these two antigens are localized in different cell populations. Retrograde neuronal tracing utilizing fluorescent dyes (Fast blue, Fluoro-gold) combined with tyrosine hydroxylase immunohistochemistry has demonstrated that the esophagus and stomach are peripheral targets of tyrosine-hydroxylase-containing vagal visceroafferent neurons.
Similar content being viewed by others
References
Andrew BC (1956) A functional analysis of the myelinated fibres of the superior laryngeal nerve of the rat. J Physiol (Lond) 133:420–432
Auffray C, Rougeon F (1980) Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem 107:303–314
Bachmann S, Metzger R, Bunnebaum B (1990) Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle's loop in rat kidney. Histochemistry 94:517–523
Baluk P, Gabella G (1989) Tracheal parasympathetic neurons of rat, mouse and guinea pig: partial expression of noradrenergic phenotype and lack of innervation from noradrenergic fibres. Neurosci Lett 102:191–196
Czyczyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE (1991a) Expression of messenger RNAs for peptides and tyrosine hydroxylase in primary sensory neurons that innervate arterial baroreceptors and chemoreceptors. Neurosci Lett 129:98–102
Czyczyk-Krzeska MF, Bayliss DA, Seroogy KB, Millhorn DE (1991b) Gene expression for peptides in neurons of the petrosal and nodose ganglia in rat. Exp Brain Res 83:411–418
Everitt BJ, Hökfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M (1984) Differential coexistence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience 11:443–462
Forssmann WG, Ito S, Weihe E, Aoki A, Dym M, Fawcett DW (1977) An improved perfusion fixation method for the testis. Anat Rec 188:307–314
Forssmann WG, Pickel V, Reinecke M, Hock D, Metz J (1981) Immunohistochemistry and immunocytochemistry of nervous tissue. In: Heym C, Forssmann WG (eds) Techniques in neuroanatomical research. Springer, Berlin Heidelberg New York, pp 171–205
Gaudin-Chazal G, Segu L, Seyfritz N, Puizillout (1981) Visualization of serotonin neurons in the nodose ganglia of the cat. An autoradiographic study. Neuroscience 6:1127–1137
Hänze J, Kummer W, Haass M, Lang RE (1991) Neuropeptide Y mRNA regulation in rat sympathetic ganglia: effect of reserpine. Neurosci Lett 124:119–121
Helke CJ, Niederer AJ (1990) Studies on the coexistence of substance P with other putative transmitters in the nodose and petrosal ganglia. Synapse 5:144–151
Kai-Kai MA, Keen P (1985) Localization of 5-hydroxytryptamine to neurons and endoneurial mast cells in rat sensory ganglia. J Neurocytol 14:63–78
Katz DM, Black IB (1986) Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J Neurosci 6:983–989
Katz DM, Markey KA, Goldstein M, Black IB (1983) Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. Proc Natl Acad Sci USA 80:3526–3530
Kondo H (1977) Innervation of SIF cells in the superior cervical and nodose ganglia: an ultrastructural study with serial sections. Biol Cell 30:253–264
Kummer W, Habeck JO (1992) Chemoreceptor A-fibres in the human carotid body contain tyrosine hydroxylase and neurofilament immunoreactivity. Neuroscience 47:713–725
Kummer W, Gibbins IL, Stefan P, Kapoor V (1990) Catecholamines and catecholamine-synthesizing enzymes in guinea pig sensory ganglia. Cell Tissue Res 261:595–606
Kummer W, Fischer A, Kurkowski R, Heym C (1992) The sensory and sympathetic innervation of guinea pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49:715–737
Lundberg JM, Terenius L, Hökfelt T, Goldstein M (1983) High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett 42:167–172
McCutcheon FH (1964) Organ systems in adaptation: the respiratory system. In: Dill DB, Adolph EF, Wilber CG (eds) Handbook of physiology. Section 4: adaptation to the environment. Waverly, Baltimore, pp 167–191
Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR (1984) Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 12:7035–7056
Morris JL, Gibbins IL (1987) Neuronal colocalization of peptides, catecholamines and catecholamine-synthesizing enzymes in guinea pig paracervical ganglia. J Neurosci 7:3177–3130
Neuhuber WL (1987) Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst 20:243–255
Price J (1985) An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci 5:2051–2059
Price J, Mudge A (1983) A subpopulation of rat dorsal root ganglion neurones is catecholaminergic. Nature 301:241–243
Sachs L (1974) Angewandte Statistik. Springer, Berlin Heidelberg New York
Sourkes TL (1977) Enzymology of aromatic amino acid decarboxylase. In: Usdin E, Weiner N, Youdim MBH (eds) Structure and function of monoamine enzymes. Dekker, New York, pp 477–496
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kummer, W., Bachmann, S., Neuhuber, W.L. et al. Tyrosine-hydroxylase-containing vagal afferent neurons in the rat nodose ganglion are independent from neuropeptide-Y-containing populations and project to esophagus and stomach. Cell Tissue Res 271, 135–144 (1993). https://doi.org/10.1007/BF00297551
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00297551